3192
Dynamic imaging of hyperpolarized xenon-129 uptake in the human brain with spiral MRI1University Of Sheffield, Sheffield, United Kingdom, 2GE Healthcare, Munich, Germany
Synopsis
In this study we explore the use of continuous 2D spiral k-space sampling to dynamically image the uptake of inhaled hyperpolarized 129Xe dissolved in human brain tissue. The sliding window reconstruction enables the monitoring of xenon uptake dynamics in the gray matter at high temporal resolution. Dynamic 129Xe brain MRI may be useful in pathologies related to cerebral perfusion and may provide insight in to blood brain barrier permeability.
Introduction
Recent developments with MR imaging of hyperpolarized (HP) 129Xe dissolved in the human brain1-3 have generated interest in xenon as a novel MR contrast agent for assessment of brain perfusion and blood-brain-barrier (BBB) function. The T1 of 129Xe dissolved in blood4 (8s) is long enough to transport sufficient signal from the lungs where the gas is inhaled to the brain tissue (grey matter5, T1 ~15s) and to ensure sufficient SNR for dissolved phase xenon brain MRI.
The highest spatial resolution images demonstrated to date with HP 129Xe human brain MRI1,3, were acquired with 2D Cartesian gradient echo acquisitions sampled over a breath-hold of 20~30s with a time duration of 8~10s between successive acquisitions. This was to allow HP 129Xe to perfuse into brain tissue in sufficient concentrations needed to provide the SNR required for the spatial resolution achieved (50x7x7 mm3). One shortcoming with this method is the lack of temporal resolution for study of xenon uptake kinetics between consecutive acquisitions. In this study, we investigate continuous 2D spiral k-space sampling with sliding window reconstruction during a 25s breath-hold to monitor xenon uptake in the brain tissue of healthy volunteers.
Methods
Hyperpolarization of 129Xe was carried out using a spin-exchange optical pumping polarizer developed in-house. A home-built transmitter birdcage RF coil along with a 4-element receiver RF coil array was used for RF transmission and signal detection respectively. In-vivo imaging was performed on a 1.5T GE HDx MRI scanner with an inhaled xenon gas dose of 1L on a healthy normal subject (M 34y). For brain imaging, HP 129Xe gas is administered by inhalation, to the lungs where it dissolves in the capillaries and is transported to the brain following the oxygen uptake pathway1. Images are acquired during and following a breath-hold to maintain a HP 129Xe gas phase signal reservoir in the lungs and it is cleared from the body by exhalation after breaking apnea.
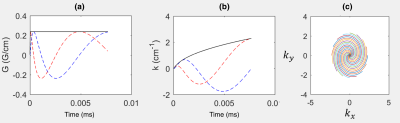
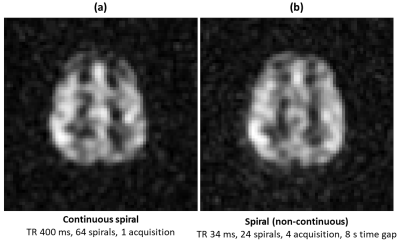
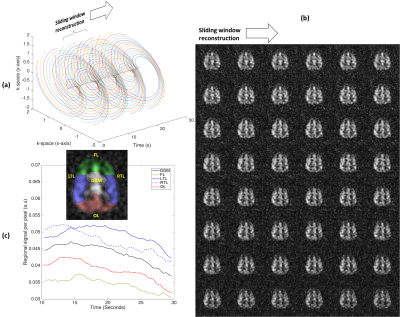
Continuous sampling of a 16-arm 2D spiral k-space trajectory started 4s after inhalation of the gas dose. The gradient waveform and the k-space sampling pattern is shown in Figure 1. A set of any 16 subsequent spiral arms can be selected for sliding window image reconstruction as shown in Figure 1(c) and 3(a). Dynamic image reconstruction with an image contrast update rate (pseudo-temporal resolution) equal to the sequence TR is therefore possible. Imaging parameters were; center frequency = 17660730Hz (194 ppm downfield of 129Xe gas phase signal), TR = 400ms, flip angle = 30°, bandwidth = 7.8kHz (oversampled to 31.25kHz), field of view = 25cm, slice thickness = 50mm and acquisition matrix = 32x32. A total of 64 spiral trajectories were thus continuously acquired. In a separate examination, for reference, another spiral k-space sampling trajectory was acquired with 24 spiral arms per acquisition with similar imaging parameters, but with TR = 34ms and flip angle = 18°. 4 images were acquired at 8, 16, 24 and 32s after the inhalation of xenon gas dose.
Results
Figure 2 shows the averaged images acquired with continuous sampling and non-continuous discrete imaging with an 8s time interval. Considering the same ROI between the images in Figure 2, we observed that the SNR (~16) was comparable with less than 10% variation, which indicates optimal utilization of polarization of 129Xe in the continuous sampling strategy such that RF depolarization effects can be separated from xenon tissue concentration uptake.
Snapshots of images from a sliding window reconstruction are shown in Figure 3(b) with refresh rate resolution of 400ms. Regional cerebral xenon uptake and tissue perfusion curves are demonstrated for the ROIs shown in Figure 3(c). The rise and fall of the signal throughout the acquisition confirms that sequence parameters such as flip angle and TR are sufficiently well tuned in order to separate RF depolarization and the underlying T1 decay of 129Xe in grey matter5 (~15s) from xenon blood-to-tissue uptake to allow the future regional study of uptake of xenon in human brain tissue.
Discussion
Xenon is chemically inactive6 and diffuses passively across the BBB in to brain tissue based on local brain physiology and xenon’s solubility. In comparison to the previous studies1,3 the proposed method allows temporal monitoring of xenon uptake in brain tissue and could be used in the future for quantification of regional cerebral blood flow and studies of BBB physiology. The method could be of interest in pathologies related to both cerebral perfusion such as stroke and stenosis, and intact-barrier disorders such as edema and arterial plaque. In addition, the passive nature of tracer would serve as a baseline such as to cross-reference with oxygen, water or glucose uptake driven by metabolism, electrolytic balance or both.Acknowledgements
This work was funded by the Engineering and Physical Sciences Research Council (EPSRC), National Institute for Health Research (NIHR) and Medical Research Council (MRC). This report is an independent research supported by the NIHR Research Professorship.References
- Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging. Radiology;0(0):162881.
- Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM. High resolution spectroscopy and chemical shift imaging of hyperpolarized (129) Xe dissolved in the human brain in vivo at 1.5 tesla. Magn Reson Med 2016;75(6):2227-2234.
- Li T, al. e. Using Hyperpolarized 129Xe MRI to Detect Impaired Cerebral Perfusion in Human Brain with Alzheimer’s Disease. Proc Intl Soc Mag Reson Med 25 P 0487 2017.
- Norquay G, Leung G, Stewart NJ, Tozer GM, Wolber J, Wild JM. Relaxation and exchange dynamics of hyperpolarized (129) Xe in human blood. Magn Reson Med 2015;74(2):303-311.
- Kilian W, Seifert F, Rinneberg H. Dynamic NMR Spectroscopy of Hyperpolarized 129Xe in Human Brain Analyzed by an Uptake Model. Magnetic Resonance in Medicine 2004;51(4):843-847.
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews Neuroscience 2006;7(1):41-53.
Figures