3183
A novel minimally invasive, image-guided neurablation technique using MRI - MINIMA1Centre for Advanced Biomedical Imaging, UCL, London, United Kingdom, 2Department of Medical Physics and Biomedical Engineering, UCL, London, United Kingdom
Synopsis
During tumour resection the goal is to remove a discrete region of cancerous tissue causing minimal damage to surrounding healthy tissue. Therefore, there is a demand for minimally invasive techniques, alongside MR imaging, for precise location of the tumour boundary. Presented here is the development of a minimally invasive, image-guided neurosurgery technique, whereby the position of an untethered surgical implant can be controlled and imaged in real time using an MRI scanner. We have demonstrated image-guided, precise movement of millimetre sized magnetic spheres inside ex vivo brain tissue by controlling the magnetic field gradients inside an MRI scanner.
Introduction
The outcome of benign brain tumours depends on whether the surgeon is able to safely remove all of the tumour. Furthermore, removal of cancerous tissue can lead to damage of the surrounding normal tissue. Presented here is the development of a novel minimally invasive, image guided neurosurgical technique using an MRI scanner, know as MINIMA, to enable precise ablation of the affected tissue. This technique uses a millimetre sized magnetic sphere which can be inserted into the brain superficially, then moved into the tumour via a magnetic force by controlling the magnetic field gradients of the MR system. As MRI is already used to detect tumour boundaries, the position of the sphere can be moved and assessed in a real-time feedback loop to ensure it is not moved into surrounding healthy tissue. As the sphere is guided through the tumour it can either be heated to induce hyperthermia, or deposit a therapeutic agent.Method
Experimental data were acquired using a 9.4T Varian scanner. Chrome steel spheres of 0.5mm and 1mm diameters were placed in a 22x25x25mm phantom filled with 0.125% agar to assess precision of movement in 3 orthogonal directions. Assessment of movement within tissue was performed using a 2mm steel sphere inserted into ex vivo pig brain tissue at 37°C. Magnetic gradients were applied using a standard preclinical gradient set with the following parameters: Gradient strength = 200-500mT/m, on/off duty cycle = 2/7ms, loop repetitions = 500 prior to short echo time imaging.Results
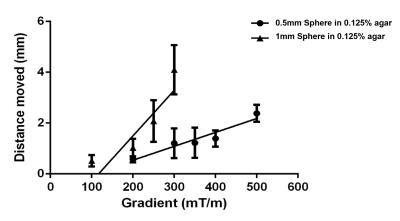
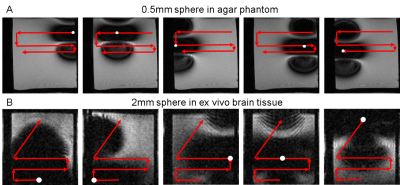
The precision of image-guided control was assessed by moving a single magnetic sphere along a predetermined path in 0.125% agar and subsequently brain tissue. Figure 1 shows that changing the strength of the magnetic field gradient from 100-500mT/m gives a range of movement between 0.5-2mm for a 0.5mm diameter sphere and 0.5-4mm for a 1mm diameter sphere with an on/off duty cycle of 2/7ms and 500 loop repetitions. In Figure 2, axial images show the position of a 0.5mm magnetic sphere (indicated by a white circle) in a 0.125% agar phantom and a 2mm sphere in ex vivo brain tissue at successive time points when moved along a predetermined path (red arrows) in the read out and phase encoding directions. The 0.5mm sphere was moved in 2mm incremental movements whereas the 2mm sphere varied between <0.5mm to 2mm due to different tissue structures within the sample. For movement in all three dimensions (read out, phase encoding, slice selective) a minimum size of 1mm was needed in 0.125% agar and 2mm for ex vivo brain tissue. All spheres moved when the directional gradients were applied and remained stationary in the sample during image acquisition and when left idle.Discussion
Our results show that it is possible to manipulate the position of a magnetic sphere within tissue with a high degree of control using a preclinical MR system. To date, magnetic targeting experiments using MRI gradient sets have focused on influencing movement in the vasculature to enhance delivery of micron size particles1 or SPION loaded cells to a target area2,3. Martel et al have also demonstrated the feasibility of moving millimetre sized steel balls within the artery of a living swine with real time automated feedback4. However, this is the first demonstration that sufficient forces can be generated to move a 2mm sized magnetic sphere through brain tissue. Further optimisation of the imaging sequence is still needed to improve accuracy when assessing the position of the sphere. Nonetheless, this research opens up the possibility of using MRI to both image and, with precision (order of 1mm), guide the surgery, ablation or drug delivery.Conclusion
We have developed a new minimally invasive surgical technique using untethered magnetic spheres that can be controlled and assessed in real time using an MRI scanner-MINIMA. We have shown that it is possible to move a magnetic sphere through ex vivo brain tissue with tuneable control and precision. Further work is needed to improve accuracy in locating the sphere in tissue, either through optimising the imaging sequence or post processing techniques. Investigations into both heating the sphere and delivering therapeutic agents are ongoing.Acknowledgements
No acknowledgement found.References
(1) Pouponneau P et al., Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation, Biomaterials, 2011; 32: 3481-3486
(2) Riegler J, Lau KD, Garcia-Prieto et al., Magnetic cell delivery for peripheral arterial disease: A theoretical framework, Med Phys, 2011; 38: 3932
(3) Munitta Muthana, Aneurin J. Kennerley, Russell Hughes et al., Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting, Nature Comms. 2015; 10: 1038
(4) Martel S et al, Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system, Appl. Phys. Lett, 2007; 90: 114105
Figures