3165
Resting-state fMRI study of brain activation using low-intensity repetitive transcranial magnetic stimulation in rats.1Experimental and Regenerative Neurosciences, School of Biological Sciences, The University of Western Australia, Crawley, Australia, 2School of Veterinary and Life Sciences, Murdoch University, Murdoch, Australia, 3Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, Nedlands, Australia, 4Perron Institute for Neurological and Translational Research, Perth, Australia
Synopsis
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique used to treat many neuropsychiatric conditions. However, the mechanisms underlying its mode of action are still unclear. This is the first rodent study using resting-state fMRI to examine low-intensity (LI) rTMS effects, in an effort to provide a direct means of comparison between rodent and human studies. Our study shows that similar to human rTMS, 10 Hz LI- rTMS alters the resting brain activity of rats directly at the site of stimulation (e.g. cortex) as well as in remote but inter-connected brain regions (e.g. hippocampus).
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique that has shown therapeutic potential in a range of neurological and psychiatric conditions.1-10 Interleaving resting-state functional MRI (rs-fMRI) and rTMS in humans has allowed for direct visualisation of the effects of rTMS on resting-state neuronal networks (RSN), e.g. the default mode network. However, there have been no reports of animal studies using those same techniques. Because rodents are widely used as preclinical models of various neuropsychiatric conditions, a thorough understanding of how rTMS affects the rodent RSN is of particular importance for both interpreting rodent rs-fMRI data and translating findings between animal models and humans. To our knowledge, this is the first rodent study to use rs-fMRI to examine the effects of low-intensity (LI) rTMS, in an effort to provide a direct means of comparison between rodent and human studies.Methods
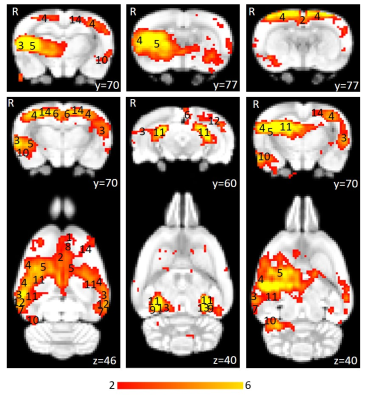
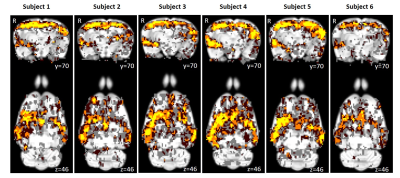
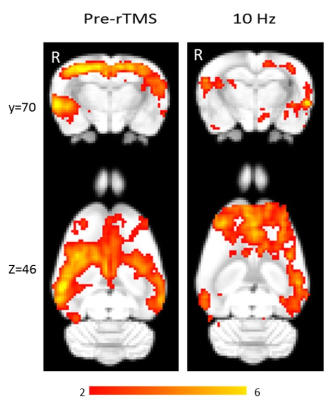
Using isoflurane-anaesthetised Sprague-Dawley rats, rs-fMRI data were acquired at 9.4 T before and immediately after 10 minutes of sham or active 10 Hz LI-rTMS over the right hemisphere using custom-built round coil. Each animal was scanned at four timepoints and group-ICA was used to identify the RSN (Figure 1). The data was used for two separate analyses. First, we investigated the reproducibility of the RSN over time and between subjects using pre-stimulation group-ICA results (Figure 2). Post-stimulation group-ICA components were then compared to pre-stimulation RSN to investigate the effects of 10 Hz LI-rTMS (Figure 3).Results and Discussion
The reproducibility maps illustrate that the middle part of the clusters show overlap for at least three timepoints while the border of the clusters represents data from only one or two timepoints. Most of the scatter and single-voxel correlations come from single sessions. This indicates that the central resting-state activity is reproducible, even when the rs-fMRI data acquisition is separated by a week or more. The same pattern is seen in each of the six animals, demonstrating that the data is also reproducible between subjects.
Group-ICA revealed clear changes in the synchronised neuronal activity following 10 Hz LI-rTMS. An ipsilateral decrease in the synchrony of the resting activity of the somatosensory cortex was observed. Our finding is compatible with those of a human fMRI study by Schneider, et al.11 showing an increase in activity of the targeted brain region when 5 Hz rTMS (considered excitatory and have roughly equivalent effects to 10 Hz12) was applied over the left primary somatosensory cortex.
Applying high-frequency rTMS to the lesioned hemisphere for the treatment of patients with stroke has been found to improve ipsilesional hemispheric excitability and hence improving motor rehabilitation. For example, when 5 Hz (high-frequency) rTMS was applied ipsilesionally in a stroke study, a bilateral increase in motor connectivity was found.13 In accordance with this study, we also found a bilateral increase in the synchrony of resting activity in the motor cortex following 10 Hz stimulation.
While the proximal changes in the RSN may reflect direct stimulation of those brain regions, the very low intensity of the magnetic field applied in LI-rTMS means that any change in the activity of the hippocampus would likely be indirect and due to the modulation of functionally connected regions. We detected an ipsilateral decrease in the synchronised activity of the hippocampus following 10 Hz stimulation. In humans, an ipsilateral change in the hippocampus was detected following high-frequency (20 Hz) stimulation to the left lateral parietal cortex of healthy adults to non-invasively enhance the targeted cortical-hippocampal networks and study their role in associative memory.14 An ipsilateral change in the hippocampus was detected following multiple-session stimulation and the increased functional connectivity was correlated with improved associative memory performance. Hence, the present results display a correlation profile that is coherent with what is known about the effect of high-frequency rTMS on the hippocampus in the literature.
Conclusion
The present findings demonstrate that 10 Hz LI-rTMS modulates functional links within the rat RSN, and the observed changes are similar to those described in humans following high-frequency rTMS. Nonetheless, the precise mechanisms generating these changes remain to be elucidated. To better understand the mechanisms underlying the reported clinical benefits of rTMS in different neuropsychiatric conditions, relevant animal models could be used to link the rTMS-induced changes in RSN to the changes in symptoms. Subsequent invasive techniques can then be used to explore those effects in greater detail and provide information about how observed functional changes reflect those detected at a molecular and cellular level. This study provides a framework to use neuroimaging to explore how LI-rTMS affects the rodent RSN, promoting evidence-based translation to human treatments.Acknowledgements
The authors thank Clare Auckland (Murdoch University) and Marissa Penrose for their technical assistance and Dr Andrew Mehnert for his help with the co-registration and analysis of the fMRI data. The authors acknowledge the facilities, and the scientific and technical assistance of the National Imaging Facility at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments. This research was funded by The University of Western Australia and the School of Veterinary and Life Sciences at Murdoch University. BJS is supported by a Forrest Research Foundation Scholarship, an International Postgraduate Research Scholarship, and a University Postgraduate Award. JR was supported by an NHMRC Senior Research Fellowship. KWF is an Australian National Imaging Facility Fellow, a facility funded by the University, State and Commonwealth Governments.References
1. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: Focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119–130.
2. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. J Clin Psychiatry. 2014;75(5):477–489.
3. Dlabač-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: Review and meta-analysis. J Clin Psychiatry. 2010;71(4):411–418.
4. Jaafari N, Rachid F, Rotge J-Y, et al. Safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorder: A review. World J Biol Psychiatry. 2012;13(3):164–177.
5. Clark C, Cole J, Winter C, et al. A review of transcranial magnetic stimulation as a treatment for post-traumatic stress disorder. Curr Psychiatry Rep. 2015;17(10):1–9.
6. Arias-Carrion O. Basic mechanisms of rTMS: Implications in Parkinson's disease. Int Arch Med. 2008;1(1):2.
7. Machado S, Arias-Carrion O, Paes F, et al. Effects of repetitive transcranial magnetic stimulation on dystonia: An overview. Am J Neuroscience. 2011;2(1):5–16.
8. Soleimani R, Jalali MM, Hasandokht T. Therapeutic impact of repetitive transcranial magnetic stimulation (rTMS) on tinnitus: A systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2015;273(7):1663–1675.
9. Pereira LS, Müller VT, da Mota Gomes M, et al. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review. Epilepsy Behav. 2016;57(Pt A):167–176.
10. Corti M, Patten C, Triggs W. Repetitive transcranial magnetic stimulation of motor cortex after stroke: A focused review. Am J Phys Med Rehabil. 2012;91(3):254–270.
11. Schneider SA, Pleger B, Draganski B, et al. Modulatory effects of 5Hz rTMS over the primary somatosensory cortex in focal dystonia—An fMRI-TMS study. Mov Disord. 2010;25(1):76–83.
12. Wilson MT, St George L. Repetitive transcranial magnetic stimulation: A call for better data. Front Neural Circuits. 2016;10:57.
13. Li J, Zhang X-W, Zuo Z-T, et al. Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: An fMRI study. CNS Neurosci Ther. 2016;22(12):952–960.
14. Wang JX, Rogers LM, Gross EZ, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–1057.
Figures