3163
Assessment of cerebral infarction due to intracranial arterial stenosis in the human brain using hyperpolarized xenon-129 MRIMadhwesha Rao1, Graham Norquay1, Neil J Stewart1, Nigel Hoggard1, Paul D Griffiths1, and Jim M Wild1
1University Of Sheffield, Sheffield, United Kingdom
Synopsis
In this pilot study we evaluate the feasibility of hyperpolarised 129Xe brain perfusion MR imaging to evaluate brain tissue perfusion patho-physiology in a subject with established intracranial arterial stenosis with collateralized blood flow. The 129Xe brain perfusion images from the patient exhibit regions of signal void which correspond to the infarcted region observed on T2 weighted MRI. Imaging hyperpolarized 129Xe dissolved in the brain tissue is a direct method of imaging perfusion of the tissue itself and we observe different patterns of cerebral perfusion than those measured with arterial spin labeling.
Introduction
Widely used clinical imaging methods for the assessment of brain perfusion are perfusion CT and contrast-enhanced MRI1, both of which require the use of chelated contrast agents that do not cross the intact blood brain barrier (BBB). CT requires significant doses of ionizing radiation and gadolinium-chelated contrast agents have potential risks associated with renal complications2 and intracranial deposition3. MRI-based arterial spin labeling (ASL)4 studies do not use injected exogenous agents, however, require averaging over several minutes5. Recently, studies have shown the possibility of imaging the uptake of hyperpolarized (HP) 129Xe in to brain tissue as it crosses the intact BBB in both healthy normal subjects6,7 and patients with Alzheimer’s disease8. In this study, we investigate the sensitivity of HP 129Xe brain MRI for the evaluation of intracranial arterial stenosis (IAS) with collateralized blood flow where the blood supply to brain tissue is delayed rather than deprived.Methods
129Xe was hyperpolarized using a home-built spin-exchange optical pumping polarizer capable of generating 1 L doses of 129Xe polarized to ~35% in 20 minutes. In-vivo imaging was performed on a 1.5 T GE HDx MRI scanner using a home-built 4 element receiver coil array as shown in Figure 1. Upon inhalation, xenon dissolves in the pulmonary vasculature and is transferred to the brain through the systemic blood circulation with a long dissolved phase T1 (8 s (9)). Subsequently, HP 129Xe dissolved in the brain tissue can be detected. MR imaging was performed with the following parameters; spoiled gradient echo, center frequency = 17660800 Hz (198 ppm downfield of 129Xe gas phase signal), TE = 1.7 ms, TR = 34 ms, flip angle = 12.5°, bandwidth = 4 kHz, field of view = 22 cm, slice thickness = 50 mm and acquisition matrix = 32 x 32. Three images were acquired during a single breath hold at 8 s, 16 s and 24 s after the inhalation of the xenon gas dose of 1 L. The images were averaged. 1 volunteer with a pre-established pathological condition of IAS with collateralization (Male 52 y) and 3 healthy volunteers (Male 33 y, 26 y and 31 y) were imaged. HP 129Xe brain imaging was compared with images from time-of-flight angiography, T2 weighted imaging and pseudo-continuous Arterial Spin Labelling (pCASL) with recommended imaging parameters5, performed on 3.0 T Philips Ingenia MRI Scanner.Results
Figures 2 and 3(a) are the images from the volunteer with IAS with collateralization. Figure 2(a), (b) and (c) are images from the time-of-flight, T2 weighted and pCASL, which indicate; the location of stenosis, infarct (watershed) region in the brain and atypical ASL perfusion due to stenosis. Figure 3(a) and (b) shows HP 129Xe MR images from the volunteer with IAS with collateralization and a healthy normal subject for reference. Comparing these images, we observe a region of signal void in the image of the volunteer with IAS (Figure 3 (a)), which is not seen in the image of healthy normal subject (Figure 3(b)), and this location corresponds to the infarcted region on T2 weighted MRI in Figure 2(b).Discussion
Xenon crosses the BBB passively and diffuses into the extra-vascular tissue compartment, and the signal is weighted towards 129Xe dissolved in grey matter6,7. Thus, MR imaging of 129Xe dissolved in the brain is a direct method of imaging actual delivery of xenon (gas) to the tissue rather than intravascular perfusion alone6. The regions of signal void in the HP 129Xe brain image indicate poor regional uptake of xenon possibly due to impaired capillary exchange and tissue damage in the watershed region, and we believe this may provide added sensitivity to assess tissue viability. In subjects with collateralization, the blood supply to the infarct region may be delayed rather than totally deprived. This mechanism may also contribute to the weak regional signal observed here due to a combination of factors arising from the higher residency time of HP 129Xe in the blood before being delivered to the tissue. These include shorter T1 in the dissolved blood9 compartment ( 8 s) when compared to grey matter10 (15 s), and reduction in quantity due to xenon diffusing away from the blood vessel.Conclusion
We have demonstrated the feasibility of performing HP 129Xe brain MRI in a clinically stable subject with established cerebral pathology. Hyperpolarized 129Xe MRI is an injection free means of directly imaging the perfusion of viable cerebral tissue and thus provides novel and distinct contrast to established brain imaging methods.Acknowledgements
This work was funded by the Engineering and Physical Sciences Research Council (EPSRC), National Institute for Health Research (NIHR) and Medical Research Council (MRC). This report is an independent research supported by the NIHR Research Professorship.References
- Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke 2005;36(9):e83-99.
- Ledneva E, Karie S, Launay-Vacher V, Janus N, Deray G. Renal safety of gadolinium-based contrast media in patients with chronic renal insufficiency. Radiology 2009;250(3):618-628.
- McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015;275(3):772-782.
- Wolf RL, Detre JA. Clinical Neuroimaging Using Arterial Spin-Labeled Perfusion MRI. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2007;4(3):346-359.
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine 2015;73(1):102-116.
- Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging. Radiology;0(0):162881.
- Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM. High resolution spectroscopy and chemical shift imaging of hyperpolarized (129) Xe dissolved in the human brain in vivo at 1.5 tesla. Magn Reson Med 2016;75(6):2227-2234.
- Li T, al. e. Using Hyperpolarized 129Xe MRI to Detect Impaired Cerebral Perfusion in Human Brain with Alzheimer’s Disease. Proc Intl Soc Mag Reson Med 25 P 0487 2017.
- Norquay G, Leung G, Stewart NJ, Tozer GM, Wolber J, Wild JM. Relaxation and exchange dynamics of hyperpolarized (129) Xe in human blood. Magn Reson Med 2015;74(2):303-311.
- Kilian W, Seifert F, Rinneberg H. Dynamic NMR Spectroscopy of Hyperpolarized 129Xe in Human Brain Analyzed by an Uptake Model. Magnetic Resonance in Medicine 2004;51(4):843-847.
Figures
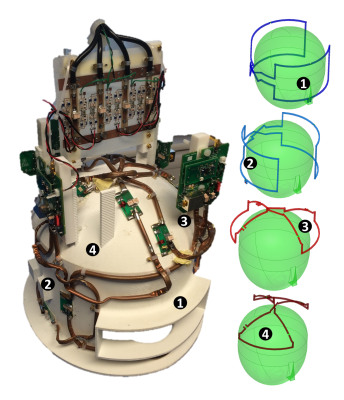
Figure 1: Home-built 4 element receive only RF coil for HP 129Xe brain MRI at 1.5 T (17.65 MHz). Left: Photograph; Right: Illustration indicating individual elements. (Annotated number correspond individual elements of coil between the photograph and illustration).
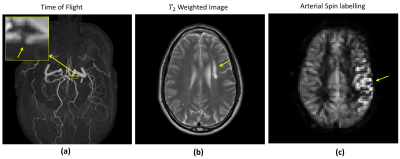
Figure 2: Conventional MR images of a volunteer (male 52 years) with intracranial arterial stenosis with collateralization. (a) Time-of-flight, (b) T2 weighted image and pseudo-continues arterial spin labelling (pCASL), performed on 3.0 T. Arrows indicate location of stenosis, infarct region and atypical ASL perfusion in (a),(b) and (c) respectively.
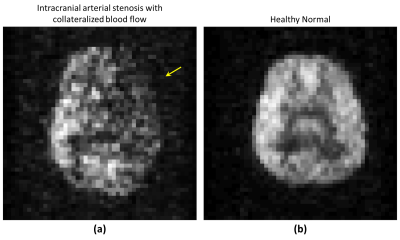
Figure 3: Hyperpolarized 129Xe
brain MRI of (a) volunteers with intracranial arterial stenosis and (b) healthy
normal subject, performed on 1.5 T scanner. Arrow in (a) is pointing to region
of signal void indicating poor regional uptake of xenon, not seen in healthy
normal subject in (b).