3160
The impact of age and sex on mouse brain stiffness measured with Magnetic Resonance Elastography1Institute of Neuroradiology, University Medical Center Goettingen, Goettingen, Germany, 2Department of Radiology, Brigham and Women's Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Department of Biomedical Engineering, Boston University, Boston, MA, United States, 5Ann Romney Center for Neurologic Diseases, Brigham and Women's Hospital, Boston, MA, United States, 6Department of Radiological Imaging, Imaging Sciences & Biomedical Engineering Division, King's College London, London, United Kingdom
Synopsis
Aging is accompanied by neurodegeneration, which affects the cerebral biomechanical properties. We investigated the impact of age and sex on mouse brain stiffness using magnetic resonance elastography (MRE). Repeated MRI and MRE exams were performed on 5 male and 5 female healthy C57BL/6 mice over 14 months. A significant decrease of the viscoelastic modulus |G*| was observed, while the phase angle Y remained unaltered. Grey and white matter exhibited significant differences in |G*| and Y. Sex differences were observed in the cortex at 11 months. This is relevant for future cerebral MRE studies on mice.
Introduction
Aging is accompanied by a progressive degradation of brain tissue due to an ongoing loss of glial cells and neurons1. This process affects the structural matrix of the brain and has an impact on its biomechanical properties. Studies in healthy volunteers have shown that aging leads to a softening of brain tissue2–4. One group additionally reported differences in stiffness between male and female brains2, but other studies have controversial results regarding sex differences in brain viscoelasticity3,4. An opposing trend has been observed in adolescent mice, where ongoing brain maturation caused an increase in brain stiffness5,6. The aim of this project was to investigate the impact of age and sex on mouse brain stiffness measured globally and regionally with magnetic resonance elastography (MRE).Methods
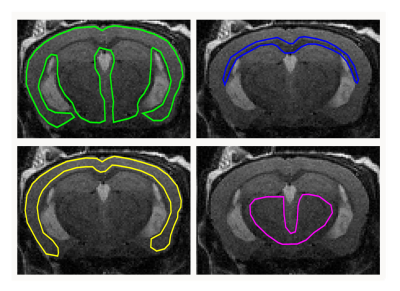
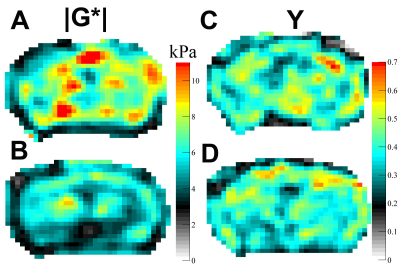
All experiments were approved by the institutional animal care and use committee. Ten healthy C57BL/6 mice (5 female/5 male) were included. Longitudinal T2w MRI and 3D MRE images were acquired with a 7 T small animal scanner at 8, 11 and 14 months of mice age. Regions of interest (ROIs; Fig. 1), which were defined on T2w images and copied to elastograms, cover the whole brain, the corpus callosum (as a representative structure of white matter), the cortex (representative of cortical grey matter) as well as the basal ganglia and thalamus (representative of deep grey matter). Mean and standard deviation of two MRE parameters, the viscoelastic modulus |G*| and the phase angle Y, were calculated for each ROI (|G*| = √(Gd2 + Gl2); Y = 2/π atan(Gl/Gd); Gd = elastic shear modulus, Gl = loss shear modulus). |G*| informs about tissue rigidity, while Y indicates whether elastic (Y ≈ 0) or viscous (Y ≈ 1) properties prevail. Repeated measures two-way ANOVA with Tukey’s post-test for multiple comparisons were performed to analyze i) regional differences in brain stiffness over time across the whole cohort and ii) stiffness of grey and white matter separately over time and between sexes.Results
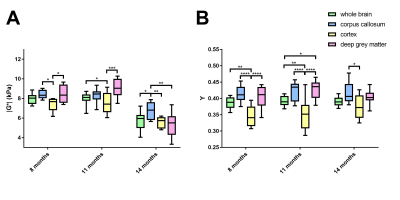
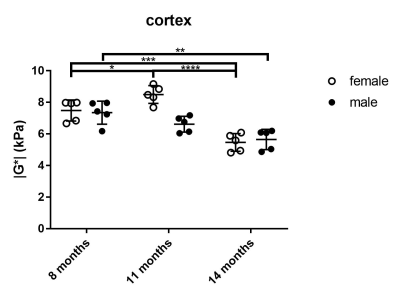
The viscoelastic modulus |G*| of the whole brain decreased significantly over time (Fig. 2A, B; 3A). This decrease was observable for both, grey and white matter structures (p < 0.0001). However, grey and white matter exhibited significantly different values of the viscoelastic modulus and the phase angle (Fig. 3). There were no significant differences between male and female mice in |G*| of the whole brain (p = 0.78), the corpus callosum (p = 0.24), or the deep grey matter (p = 0.78). When comparing sexes, the cortex of female mice was significantly stiffer than in male mice at an age of 11 months (p = 0.03; Fig. 4). In contrast to the viscoelastic modulus, the phase angle Y of all regions analyzed remained constant over time (p = 0.15; Fig. 2C, D; 3B). Additionally, the phase angle was similar for male and female animals (p > 0.05 for all ROIs).Discussion
This study is the first to investigate the impact of physiological aging on mouse brain stiffness using MRE. The observed decrease of the viscoelastic modulus with a concomitantly stable phase angle means that both elastic and viscous components of brain tissue are affected by aging and change temporally in synchrony. Our results are in line with findings reported in human healthy volunteers. Sack et al. ascribed a similar decrease in viscoelasticity and an unaltered phase angle to a decreasing neuron-glia ratio with age2, as glial cells have been found to be softer than neurons7. Interestingly, there was a difference in cortical stiffness between male and female mice at 11 months. This may indicate a sexually dimorphic pattern of neurodegeneration, which has already been demonstrated in the cortex of rats8. The ROIs used in our study were based on anatomical structures and we found differences in |G*| and Y between grey and white matter. Previous studies investigating the stiffness of grey and white matter in humans have been controversial, as some reported grey matter to be stiffer9, whereas others found white matter stiffness to be higher10. Healthy mice of various ages and different sexes are regularly used as controls in studies assessing stiffness of various pathologies with MRE. Therefore, the influence of physiological aging processes demonstrated here should be considered in such studies.Conclusion
Physiological aging impacts mouse brain stiffness. This is of relevance for future cerebral MRE studies on mice.Acknowledgements
We acknowledge grant support from NIH R21 EB020757, from European Commission Horizon 2020 proposal 668039 and from Boston University College of Engineering and the Brigham and Women’s Hospital Department of Radiology. K.S. received funding from the German Research Foundation (DFG, SCHR 1542/1-1).References
1. Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278(5337):412-419.
2. Sack I, Beierbach B, Wuerfel J, et al. The impact of aging and gender on brain viscoelasticity. NeuroImage. 2009;46(3):652-657. doi:10.1016/j.neuroimage.2009.02.040.
3. Sack I, Streitberger KJ, Krefting D, el al. The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PloS One. 2011;6(9):e23451. doi:10.1371/journal.pone.0023451.
4. Arani A, Murphy MC, Glaser KJ, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage. 2015;111:59-64. doi:10.1016/j.neuroimage.2015.02.016.
5. Schregel K, Wuerfel E, Garteiser P, et al. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc Natl Acad Sci U S A. 2012;109(17):6650-6655. doi:10.1073/pnas.1200151109.
6. Munder T, Pfeffer A, Schreyer S, et al. MR elastography detection of early viscoelastic response of the murine hippocampus to amyloid β accumulation and neuronal cell loss due to Alzheimer’s disease. J Magn Reson Imaging. April 2017:n/a-n/a. doi:10.1002/jmri.25741.
7. Lu YB, Franze K, Seifert G, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci U S A. 2006;103(47):17759-17764. doi:10.1073/pnas.0606150103.
8. Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23(4):579-588.
9. Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21(7):755-764. doi:10.1002/nbm.1254.
10. Kruse SA, Rose GH, Glaser KJ, et al. Magnetic resonance elastography of the brain. NeuroImage. 2008;39(1):231-237. doi:10.1016/j.neuroimage.2007.08.030.
Figures