3159
Regional Changes in Brain Stiffness with Age Assessed with MR Elastography1Department of Radiology, University of Yamanashi, Yamanashi, Japan, 2Department of Radiology, Juntendo University, Tokyo, Japan, 3Department of Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
Various MR-assessed quantities change with age in the brain, including T2, diffusion, and fractional anisotropy. MR elastography (MRE) is a new technique that can measure the stiffness of tissue and is now widely used in the clinic for assessing hepatic fibrosis. Recently, it has become feasible to perform brain MRE as well. In this study, we showed that the stiffness of the brain, measured using MRE, decreased with age, especially in the parietal lobes and the sensory-motor area.
INTRODUCTION
Aging effects on the brain have been a topic of research for many years. In brain MRI, multiple parameters have been determined to increase/decrease with age. For example, T2 increases1), apparent diffusion coefficient increases2), fractional anisotropy of white matter decreases3), radial diffusivity of white matter increases, and mean displacement by q-space imaging increases4). MR elastography (MRE) of the brain5) is a new technique for measuring the stiffness of the brain using an external vibrator and phase-contrast MRI. Here, we assessed age-related changes of stiffness measured by MRE in healthy subjects.METHODS
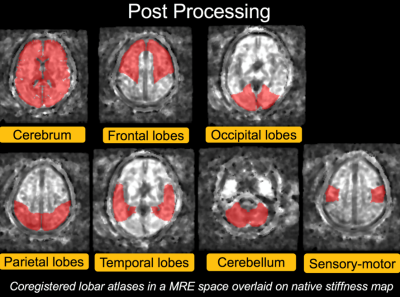
This study was approved by our institutional review board. Written informed consent was obtained from all subjects. We enrolled 50 volunteers without brain disease (5 males and 5 females in their 20s, 30s, 40s, 50s, and 60s). MRE was performed with a 3-Tesla clinical MR system (GE Discovery MR750) and a 32-channel head coil. External vibration was generated using an active driver placed outside of the MR room. The pneumatic wave was carried via a plastic tubing to a pillow-shaped passive driver which was placed under the subject’s head. The frequency of the external vibration was 60 Hz. Other MR parameters included axial, 2D, spin-echo, echo-planar imaging; TR/TE = 3600/62 ms; imaging matrix = 72×72; field of view = 24 cm; 3-mm slice thickness (with no gap); X, Y, and Z motion-sensitizing gradients; and a 5-min acquisition time. For accurate and reproducible region-of-interest (ROI) placement, a lobar atlas was coregistered to the 3D T1-weighted images that was obtained right after MRE was performed. The lobar atlas is a standard template space provided by WFU Pickatlas. (Fig.1) We compared the stiffness in each part of the brain between the subjects in their 20s (control) and those in the other age groups using the Dunnett test.RESULTS and DISCUSSION
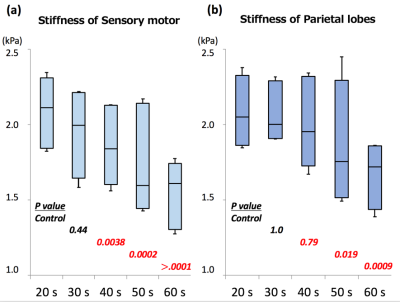
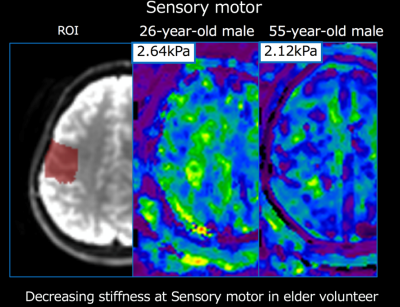
In all parts of the brain, the stiffness tended to decrease with age. The most prominent changes were observed in the sensory-motor area and the parietal lobes. The stiffness of the sensory motor was significantly decreased in subjects in their 40s (mean [standard deviation] =1.85 [0.19], p=0.038), 50s (1.69 [0.24] kPa, p<0.001) and 60s (1.56 [0.16], p<0.0001), compared to the control subjects (20s, 2.10 [0.16] kPa). The stiffness of the parietal lobe was significantly decreased in the subjects in their 50s (1.79 [0.28], p=0.019) and 60s (1.69 [0.16], p<0.001) compared to the controls (20s, 2.06 [0.16]). (Fig. 2 and 3)
In this study, we showed that the global and regional stiffness of the brain tends to decrease with age. In particular, the parietal lobe and sensory-motor area in elderly subjects was significantly softer than they were for subjects in their 20s. A previous study involving elderly subjects (>60 years old) showed brain stiffness decreases in the frontal, temporal, occipital, and parietal lobes as age increases5. Our results were consistent with these results.
CONCLUSION
MRE-assessed brain stiffness decreases with age, and the changes are most prominent in the sensory-motor area and the parietal lobes. Studies of brain stiffness in health and disease must take this age-related change into account.Acknowledgements
No acknowledgement found.References
1. Kumar R. et al. J Magn Reson Imag 2012; 35: 300-8
2. Shi C. et al. J Magn Reson Imag 2017; 46: 801-812
3. Koiweera C. et al. Neuroimage 2016; 128: 180-92
4. Fatima Z. et al. Neuroradiology. 2013; 55: 253-9
5. Arani A. et al. NeuroImage 2015; 111: 59-64
Figures