3147
Magnetic susceptibility in subcortical gray matter is associated with age-related neuropathologies: An ex-vivo QSM and pathology investigation in a community cohort of older adults1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States, 3Department of Diagnostic Radiology, Rush University Medical Center, Chicago, IL, United States, 4Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, United States, 5Department of Pathology, Rush University Medical Center, Chicago, IL, United States
Synopsis
To understand the associations of quantitative susceptibility mapping with age-related neuropathologies, it is essential to combine QSM with direct assessments of age-related brain pathologies on the same individuals. Using ex-vivo QSM for this purpose may be more advantageous than in-vivo QSM, since ex-vivo QSM overcomes several of the obstacles that complicate MRI-pathology investigations and can provide magnetic susceptibility measurements that are linked to those collected in-vivo. Therefore, our goal was to investigate the associations of magnetic susceptibility with the pathology of multiple age-related diseases by combining ex-vivo QSM with histology.
Introduction
Quantitative susceptibility mapping (QSM) is a promising tool for studying age-related brain diseases, as the pathology of these diseases may produce magnetic susceptibility shifts detectable with QSM. There are multiple findings that suggest QSM may be sensitive to iron changes related to Alzheimer’s disease (AD) and vascular dementia1,2. However, these studies are typically performed with clinical diagnosis, and are susceptible to the contributions of unidentified pathology. Clinical diagnosis cannot reliably identify coexisting pathologies, which is prevalent in older adults with a clinical diagnosis of AD. In contrast, measuring the underlying neuropathology with histology allows for an accurate assessment of disease burden, and reduces the likelihood of a misdiagnosis. With this context, the purpose of this study is to investigate the associations of magnetic susceptibility with the pathology of multiple age-related diseases by combining ex-vivo QSM with histology.Methods
Participants
223 participants were recruited from the Rush Memory and Aging Project and Religious Orders Study, two longitudinal epidemiologic clinical-pathologic cohort studies of aging3,4.
MRI Data Acquisition
The postmortem hemisphere of each participant was imaged while submerged in formaldehyde. Due to MR hardware updates throughout the two cohort studies, the postmortem tissue of each participant was imaged at one of three 3T MR scanners. For all scanners, 3D gradient-echo and 2D spin-echo data were collected with similar imaging protocols (Table 1); The voxel size of the gradient-echo and spin-echo data across all scanners was 1mm isotropic and no larger than 0.65x0.65x1.5 mm, respectively.
Quantitative Susceptibility Mapping (QSM)
Total field maps were produced for the in-vivo and ex-vivo QSM data. Local fields were calculated using PDF5. Magnetic susceptibility maps were created with MEDI6, and processed with consistency on cone data to suppress streaking artifacts7. The reference values as chosen as the mean susceptibility value of the formaldehyde solution.
Histopathological Evaluation
Within 2 weeks after ex-vivo MRI, the imaged hemisphere was dissected and evaluated for both neurodegenerative and vascular disease pathologies: AD, Lewy bodies, hippocampal sclerosis, TDP43, arteriosclerosis, atherosclerosis, cerebral amyloid angiopathy gross infarcts and microinfarcts. The evaluation was performed by a board-certified neuropathologist, who was blinded to age and clinical diagnoses.
Voxelwise correlations of magnetic susceptibility and pathology
The 223 participants were chosen based on having similar hemisphere orientations across left and right hemispheres. The spin-echo data from all participants were registered to a study-specific template generated using the “unbiased atlas construction via group-wise DRAMMS registration” tool8. For each participant, the magnetic susceptibility map was transferred to the template space by combining the corresponding transformations. The susceptibility map was median filtered and then low pass filtered.
At each voxel of the template, linear regression of the participants’ magnetic susceptibility values was conducted with the corresponding measurements for the pathologies mentioned above and the following covariates: hemisphere side, scanner, age at death, sex, years of education, and postmortem interval to fixation. The overall registration quality throughout the template was assessed using a patch-based method. Voxels with poor registration quality or with less than 130 participants in the regression were excluded. False discovery rate (FDR) was applied, and statistical significance was achieved at p<0.05 for the FDR-adjusted p-values.
Results
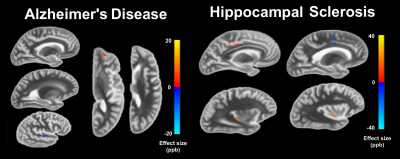
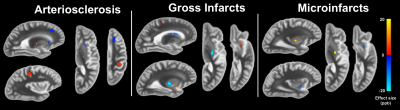
Demographic and histopathlogic information of the participants are presented in Table 2. Linear regression revealed significant associations for AD, hippocampal sclerosis, arteriosclerosis, gross infarcts, and microinfarcts (Figures 1 and 2).Conclusion
The significant associations in the caudate for AD and in the putamen for microinfarcts are in agreement with in-vivo QSM studies that rely on clinical diagnosis. The strong association with microinfarcts in the putamen may be explained by the co-occurrence of hemosiderin. These findings may be appropriate for QSM studies performed in-vivo, as ex-vivo magnetic susceptibility is linked to in-vivo magnetic susceptibility9. This study demonstrated that magnetic susceptibility shifts related to the pathology of age-related diseases can be detected by combining ex-vivo data of brain hemispheres with the pathologic correlates from histology. Significant associations were discovered for magnetic susceptibility and pathological measures of AD, hippocampal sclerosis, arteriosclerosis, gross infarcts and microinfarcts. For AD, the finding of elevated susceptibility in the caudate agrees with findings from in-vivo studies relying on clinical diagnosis. Additionally, vascular pathologies influenced the magnetic susceptibility of the subcortical gray matter. Since iron overload in the subcortical gray matter is often the focus of QSM investigations in AD, the possible contribution of vascular pathologies to QSM measurements should be considered.Acknowledgements
This work was supported by funding from National Institute on Aging (https://www.nia.nih.gov) grants P30AG10161, R01AG17917, R01AG34374, and National Institute of Neurological Disorders and Stroke (https://www.ninds.nih.gov) grant UH2NS100599, and the Illinois Department of Public Health (http://www.dph.illinois.gov) (Alzheimer’s Disease Research Fund).References
1. Acosta-Cabronero, J., Williams, G. B., Cardenas-Blanco, A., et al. In vivo quantitative susceptibility mapping (QSM) in Alzheimer’s disease. PLoS One. 2013 Nov 21;8(11):e81093.
2. Moon Y, Han SH, Moon W. Patterns of Brain Iron Accumulation in Vascular Dementia and Alzheimer's Dementia Using Quantitative Susceptibility Mapping Imaging. J Alzheimers Dis. 2016;51(3):737-45.
3. Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. 2.
4. Bennett DA, Wilson RS, Arvanitakis Z, et al. Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 2013;33 Suppl 1:S397-403.
5. Liu T, Khalidov I, de Rochefort L et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011 Nov;24(9):1129-36.
6. Liu J, Liu T, de Rochefort L et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012 Feb 1;59(3):2560-8.
7. Wen Y, Wang Y, Liu T. Enhancing k-space quantitative susceptibility mapping by enforcing consistency on the cone data (CCD) with structural priors. Magn Reson Med. 2016 Feb;75(2):823-30.
8. Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal. 2011 Aug;15(4):622-39.
9. Evia A, Kotrotsou A, Tamhane A, et al. Ex-vivo quantitative susceptibility mapping of human brain hemispheres. PLoS One. In Press.
Figures