3146
Automated Segmentation and Volumetric Analysis of the Amygdala Nuclei in Epilepsy Patients at 7 Tesla1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
The present study employs ultra-high field MRI (7 Tesla) to perform structural imaging on a group of 19 epilepsy patients and 9 healthy controls. We use automated segmentation of the amygdala to derive volumes of constituent sub-nuclei. When comparing epilepsy patients and controls, we found that the volume of the right lateral nucleus was reduced compared with controls. We also found that the anterior-amygdaloid-area and the whole right amygdala approach significance for reduced volume. These are the first in vivo findings that indicate particular nuclei are affected in epilepsy patients.
Introduction
The amygdala’s location in the temporal lobe implicates its role in seizure activity, and the structure’s role in epilepsy symptomatology is well established [1]. Hippocampal changes in epilepsy have been extensively studied, however amygdalar alterations are less understood [1,2]. Numerous imaging studies have found global volume reduction in the amygdala of epilepsy patients [1]. However, because amygdala nuclei have functionally distinct roles, it is also important to quantify amygdalar changes on a nuclear scale. For example, histopathological studies have indicated that amygdalar damage from seizure activity may show nuclear specificity with certain nuclei, such as the lateral nucleus, being more susceptible than others, like the central nucleus [1,3]. Here we report the first in vivo findings for nuclei-specific changes that occur in the amygdala in epilepsy patients.Methods
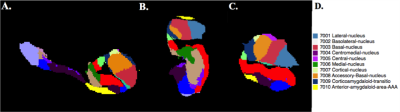
Nineteen epilepsy patients and 9 healthy controls were scanned using a 7T whole body scanner (Siemens Magnetom) under an IRB-approved protocol. The MRI protocol consisting of a T1-weighted MP2RAGE sequence (0.7 mm isotropic resolution, TE =5.1ms) and a T2-weighted TSE (2mm slice thickness, coronal oblique with 0.4mmx0.4mm in plane resolution, TE =69ms). Segmentation of the amygdalar nuclei was perfomed using the development version of FreeSurfer software [4] version 6.0. Segmentations were obtained using both T1-weighted MP2RAGE and T2 TSE acquisitions. The amygdala segmentation and its corresponding look-up table are shown in Figure 1. A two-tailed t-test was performed on the volumes of amygdalar nuclei in epilepsy patients and healthy controls.Results
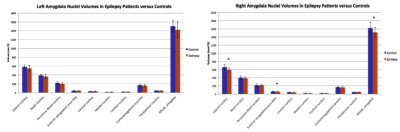
Amygdala nuclear segmentation revealed that the volume of the right lateral nucleus is reduced in epilepsy patients (Mean = 585.17 mm3 $$$\pm$$$ 48.81 mm3) compared with healthy controls (Mean = 658.51 mm3 $$$\pm$$$ 69.45 mm3), p = 0.003. Reduction of volumes in the right anterior-amygdaloid-area (AAA) and the entire right amygdala approached significance (p = 0.056 and p = 0.059, respectively). The volumes of the amygdalal nuclei in patients and controls can be seen in Figure 2.Discussion
This is the first study to use an automated method to segment the nuclei of the amygdala and perform volumetric comparison between epilepsy patients and controls. This analysis is potentially important because of the amygdala’s role in seizure activity [1,2]. Our finding of globally reduced amygdala volume was concordant with previous findings of reduced amygdala volume.1 However, future work is required to determine why this effect was not found bilaterally. Interestingly, histopathological studies have revealed that the lateral nucleus is the most severely affected nuclei in epilepsy patients [1,3], however this is the first imaging study to depict this in vivo. This is significant because not only is the lateral nucleus the major sensory input to the amygdala, it also has significant projections to other amygdala nuclei and regions of the cortex. This connectivity confers a central role in limbic circuitry and seizure propagation [1]. Future work will validate this study’s findings in a larger cohort, assess changes in amygdala nuclei volumes in relation to the suspected seizure onset zone, and will include tractography in order to quantify connectivity changes of amygdalar sub-regions that exist in epilepsy patients.Acknowledgements
NIH R00 NS070821
NIH R01 MH109544
Icahn School of Medicine Capital Campaign
Translational and Molecular Imaging Institute
References
1. Pitkänen, A., Tuunanena, J., Kalviainenb, R., Partanenc, K., Slamenperab, T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Research, vol. 32, no. 1-2, 1998, pp. 233–253.
2. Goddard, GV., McIntyre, DC., Leech, CK. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurology, 25 (1969), pp. 295-330.
3. Yilmazer-Hanke, D. M., Wolf, H. K., Schramm, J., Elger, C. E., Wiestler, O. D., & Blümcke, I. Subregional Pathology of the Amygdala Complex and Entorhinal Region in Surgical Specimens From Patients With Pharmacoresistant Temporal Lobe Epilepsy. Journal of Neuropathology & Experimental Neurology, 2010. 59(10), 907-920.
4. Saygin ZM & Kliemann D (joint 1st authors), Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, Stevens A, Van Leemput K, McKee A, Frosch MP, Fischl B, Augustinack JC. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage, 155, July 2017, 370-382.