Fahad Essbaiheem1, Rebecca Thornhill1, Gregory Cron1,2, John Woulfe1, Mario Kontolemos1, Beckie Manouchehri3, Nader Zakhari1, Andrew Boivin4, and Thanh Binh Nguyen1
1The Ottawa Hospital, Ottawa, ON, Canada, 2University of Ottawa, Ottawa, ON, Canada, 3Carleton University, Ottawa, ON, Canada, 4University of British Columbia, Kelowna, BC, Canada
Synopsis
In this prospective study, we have identified a combination of textural
features from contrast-enhanced T1-weighted images which can help in
differentiating tumour recurrence from post-treatment changes in patients with
high grade gliomas. The diagnostic accuracy
of textural analysis was similar or slightly higher than that of two
neuroradiologists who performed visual assessment.
Introduction
Surgical resection
followed by chemoradiation is the standard treatment of patients with high
grade gliomas. In the followup of these
patients, the presence of a newly enhancing lesion often represents a
diagnostic dilemma between tumor progression or post-treatment changes. Treatment-induced
necrosis of brain can present with a ``soap bubble`` or ``swiss cheese``
patterns on post-contrast T1 weighted images [1]. Visual qualitative analysis is subject to
interreader variability. Quantitative MRI texture features have been successfully
used to discriminate between gliomas, metastases and meningiomas [2].Purpose
To evaluate the performance
of quantitative textural features for differentiating between tumor progression
and post-treatment changes (non-progression) in patients with high grade
gliomas who developed a newly enhancing lesion following chemoradiation.Materials and Methods
This prospective study included
49 consecutive patients with a high grade glioma (grade 3- 4) treated with
chemoradiation who presented with one(multiple) newly enhancing lesion(s) seen
on gadolinium-enhanced MR. Each patient underwent a subsequent MR examination
on a 3T MR scanner (Trio,Siemens Medical Solutions), which included the
following sequences: axial T1 pre contrast (TR=280 ms, TE=2.51 ms, flip
angle=90º, voxel size= 1.1 x 0.9 x 3mm), axial VIBE T1 post contrast (Gadovist
1.0 (0.1 mmol/kg), TR=8.48 ms, TE=3.21
ms, flip angle=12º, voxel size=1 x 1 x 1mm) , axial FLAIR (TR=9710 ms, TE=93
ms, TI=2580 ms, voxel size=1.1x0.9x3 mm) and axial T2 (TR=6910ms, TE=97ms,
voxel size=0.7x0.7x3mm). Axial VIBE T1
post contrast images were resampled with a 5mm thickness. Enhancing lesions were manually segmented by
a medical student under the supervision of a neuroradiologist and saved as
volumes-of-interest (VOI) in Image J (National Institutes of Health, USA, http://rsbweb.nih.gov) for subsequent
texture analysis. We extracted textural features related to the gray-level
histogram, gray-level co-occurrence [3] and run-length matrix [4] for each 3D
VOI using MaZda® version 4.6 (P.M. Szczypiński, Institute of Electronics,
Technical University of Lodz, Poland) [5].
Lesion was classified as tumour
progression based on: (1) histopathological analysis obtained from surgical
reresection revealing predominantly viable tumour; (2) clinical deterioration
associated with progressive increase in size of the lesion(s) on followup
MRI. Lesions were classified as “non-progression”
(ie. post-treatment changes) based on (1) histopathological analysis showing
predominantly radiation necrosis; (2) stability or decrease in size on a
followup MRI without any clinical deterioration. Logistic regression identified feature sets
that would discriminate between the two groups (area under the ROC curve (AUC)
significantly greater than 0.5, P<0.05) and these sets were subsequently
used to train support vector machine (SVM) classifiers on 2/3 of the cases. (The
Unscrambler® X (v.10.1, CAMO Software). We evaluated classification
generalizability and performance using 10-fold cross-validation and then
applied the trained SVM classifiers to the remaining 1/3 of the cases to assess
accuracy.
Two neuroradiologists blinded to the final
diagnosis were asked to classify a set of 40 lesions.Results
There were 34 lesions
classified as tumor progression and 21 lesions as post-treatment changes
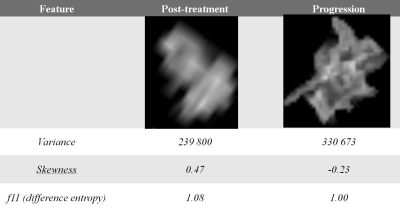
(non-progression). Length of clinical followup ranged from 5- 23 months. . Representative VOIs and textural feature values for tumor non-progression and progression are provided in Figure 1. The most
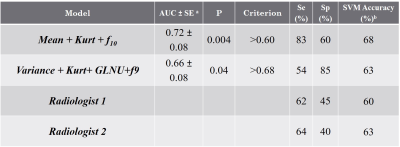
accurate logistic regression model was generated using mean pixel intensity
level, kurtosis and gray-level co-occurrence feature f10 and had an AUC of 0.72
(sensitivity=83%; specificity=60%, P= 0.004). The SVM classifier trained on
these features achieved a post-cross validation accuracy of 68% for identifying
tumors with progression(Table 1). For radiologist
1, sensitivity and specificity were 58% and 57%. For radiologist 2, sensitivity
and specificity were 69% and 64%.Conclusion
In this prospective study, we have identified a
number of quantitative textural features related to MRI gray-level variation
that may assist in identifying patients with tumor progressionAcknowledgements
This research study was supported by the Brain Tumour Foundation of Canada.References
1. Kumar
AJ, Leeds NE, Fuller GN, et al. Radiology. 2000;217(2):377–384. 2. Georgiadis P
et al. Magn Reson Imaging. 2009 Jan;27(1):120-30. 3. Haralick R, et al. IEEE
Trans Syst Man Cybern 1973. 4. Galloway MM, Comput Graph Image Proc, 1975;4(2):
172-179. 5. Szczypinski P. et al. Comput Methods Programs Biomed 2009; 94:66.