3133
Regression of Quasi-Periodic patterns diminishes BOLD functional connectivity and reveals hidden dynamic correlations1University of Antwerp, Antwerpen, Belgium, 2Emory university, Atlanta, GA, United States
Synopsis
Quasi-Periodic patterns (QPPs) represent large-scale recurring patterns in the brain, which appear as promising contributors to low frequency BOLD fluctuations. To assess the impact of QPPs on functional connectivity (FC), we used a general linear model approach to regress their contribution out of the functional images of a group of wild-type mice. We show that QPP regression diminished FC in co-active regions within the QPP, while anti-correlated regions became correlated. By calculating FC on QPP-scrubbed images, we highlight that these effects are not solely an artifact of regression. These results suggest that QPPs orchestrate dynamic correlations between resting state networks.
Introduction
Quasi-periodic patterns (QPPs) of BOLD fluctuations have been detected in mice, rats, and humans1–3. QPPs represent large-scale recurring patterns in the brain, which contribute to low frequency BOLD fluctuations4,5. Here, we developed tools to investigate how QPPs contribute to BOLD FC and apply them in a group of wild-type control mice. Using a general-linear model approach, and other tools to remove QPP contributions via image scrubbing-like strategies, we illustrate how QPP removal diminishes FC in co-active regions within the QPP, while anti-correlated regions become correlated.Methods
We employed data from a separate study in which rsfMRI was acquired in 18-month TG2576 mice (TG, N=10) and age-matched wild-type (WT, N=8) littermates on a 9.4T scanner (GE-EPI - TR:500ms; TE:16ms; 3 slices positioned on somatosensory cortex). Animals were anesthetized with isoflurane (0.4%) and medetomidine (bolus:0.3mg/kg, continuous infusion:0.6mg/kg/h). Scans were acquired 30min post-bolus. For the currently presented analysis, we investigated QPPs and their removal in the WT group.
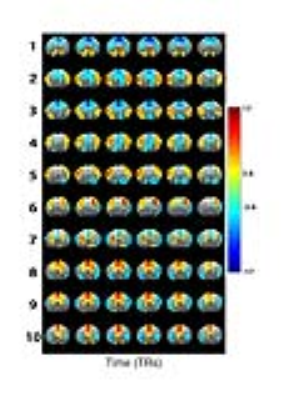
Preprocessed images (SPM12) from all subjects were concatenated to extract group-wise QPPs, without global signal (GS) regression (GSR). (Analysis performed in MATLAB2015a). QPPs are accompanied by their Sliding window Template Correlation (STC) with the reference image series (YR) from which they are derived, through averaging frames at STC peak values above an arbitrary correlation threshold (Fig.1)2. STC peak image frames were used for statistical parametric mapping of QPPs.
To assess the impact on FC, QPPs were convolved with their STCs, constructing QPP-image series (YQPP). Using the general-linear model approach, the YQPP were regressed voxel-wise out of the respective images. Fisher Z-transformed (z)FC was compared between YR and the residual images (YRES).
In alternative strategies, QPPs were removed from the functional scans by cutting their image frames centered around STC local minima and maxima. zFC was thereafter calculated (1) based on the remaining time points (YR-cut), or (2) after first filling removed time points via cubic spline interpolation (YR-spline).
To assess if the different methodologies provided complimentary outcomes on FC differences, the zFC two-sample T-test T-contrasts between YR and YR-RES were correlated with those between YR and YR-cut, and between YR and YR-spline.
Results
In the presented data, short 3s QPPs were observed that displayed strong bilateral co-activity in specific brain regions and anti-correlation with other regions. The configurations of these QPPs showed overlap with neuroanatomical locations and were visually similar to known mouse resting state networks (Fig.2).
Regression of these QPPs showed significant diminishment of FC between regions displaying matching intensities in the respective QPP, while anti-correlated regions became correlated (Fig.3A). Using a randomized STC, QPP regression did not induce any FC differences, suggesting that the regression analysis didn’t inherently induce any observed FC changes (Fig.3B). Using the alternative QPP-removal approaches, FC analysis based on YR-cut and YR-spline appeared to partially reproduce the outcome of the regression (Fig.3C-D).
A particularly interesting finding was the positive correlation between midline and lateral cortical areas, which was observed as a consequence of regressing QPPs that display anti-correlated dynamics between these regions (Fig.4A). This effect was reproduced with a lower amplitude when FC was calculated based on YR-cut, but not clearly so when using spline-interpolation (Fig.4B-C).
To compare the alternative image scrubbing-like techniques with regression, we correlated the zFC T-contrasts of before and after QPP regression (YR versus YR-RES), with the zFC T-contrasts between YR and YR-cut (Fig.5A), and between YR and YR-spline (Fig.5B). For both scrubbing methods, correlations of T-value contrasts appeared the strongest for QPPs that displayed midline with lateral cortical anti-correlation. This further emphasizes that their removal appears to reveal dynamic positive correlations.
Discussion
Regression of QPPs out of resting state functional image series diminished FC in QPP co-active regions, but increased FC in anti-correlated regions. We show that this increased FC is not solely a result of regression, but we would rather suggest that regression over-accentuates removal of anti-correlations, emphasizing dynamic positive correlations. QPP regression might thus provide novel insights into the dynamic interaction of resting state networks. It is particularly interesting to highlight the QPP-induced positive correlations between midline and lateral cortical areas, which speculatively represent mouse Default Mode like (DMN) and Task Positive like (TPN) networks3,6. Towards the future, the presented QPP removal strategies will need to be further explored and tested to validate successful removal of BOLD spatiotemporal dynamics.Acknowledgements
This work was supported, in part, by the ISMRM Research Exchange Program.
This work was further supported by the interdisciplinary PhD grant (ID) BOF DOCPRO 2014 and partially supported by funding received from: the European Union’s Seventh Framework Programme (INMiND) (grant agreement 278850, granted to AVdL), the molecular Imaging of Brain Pathohysiology (BRAINPATH) and the Marie Curie Actions-Industry-Academia Partnerships and Pathways (IAPP) program (grant agreement 612360, granted to AVdL), Flagship ERA-NET (FLAG-ERA) FUSIMICE (grant agreement G.0D7651N), and the Flemish Impulse funding for heavy scientific equipment (granted to AVdL).
References
1. Belloy, M. et al. Dynamic resting state fMRI in mice: detection of Quasi‐Periodic Patterns. Proceeding Int. Soc. Magn. Reson. Med. p.0961. (2017).
2. Majeed, W. et al. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage 54, 1140–1150 (2011).
3. Yousefi, B., Shin, J., Schumacher, E. H. & Keilholz, S. D. Quasi ‐ Periodic Patterns of Intrinsic Brain Activity in Individuals and their Relationship to Global Signal. (2017).
4. Wang, K. et al. Quasi-periodic pattern of fMRI contributes to functional connectivity and explores differences between Major Depressive disorder and control. Proc Int Soc Magn Reson Med 1683 (2016).
5. Keilholz, S. D. The neural basis of time-varying resting-state functional connectivity. Brain Connect. 4, 769–79 (2014).
6. Liska, A., Galbusera, A., Schwarz, A. J. & Gozzi, A. Functional connectivity hubs of the mouse brain. Neuroimage 115, 281–291 (2015).
Figures