3129
PROBABILISTIC MODEL-BASED FUNCTIONAL PARCELLATION OF THE PRIMARY OLFACTORY CORTEX1Radiology, Center for NMR Research, Penn State University College of Medicine, Hershey, PA, United States, 2Radiology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, Nanjing, China, 3Neurology, Penn State University College of Medicine, Hershey, PA, United States, 4Radboud University Nijmegen, Donders Centre for Brain Cognition and Behaviour, Nijmegen, The Netherlands, Nijmegen, Netherlands
Synopsis
The primary olfactory cortex (POC) is the largest recipient of olfactory bulb projections. It has a functionally versatile organization with extensive reciprocal connections to several higher-order cortical regions likely resulting in specific brain signals. Here, we propose a Bayesian model-based clustering approach, applied solely to resting state functional MRI time courses, to identify intrinsic POC functional parcellations. Results of this study suggest that multiple regions within the POC, with clear inter-hemispheric correspondence and functional relevance, can be identified using resting state fMRI data.
Abstract
Introduction: The primary olfactory cortex (POC) serves as the main input brain structure of the olfactory network. It is the largest recipient of olfactory bulb projections and is reciprocally and extensively connected with several higher-order cortical regions, including the prefrontal, amygdaloid, perirhinal and entorhinal cortices [1]. This topographical organization of the POC is likely to result in functionally specific brain signals which can be examined using functional magnetic resonance imaging (fMRI) and clustering techniques. In this study, we combined a recently developed model-based Bayesian clustering technique with resting state fMRI data (i.e., time courses) to identify intrinsic POC parcellations [2]. We expected the probabilistic model-based clustering technique to identify data-driven parcellations within the POC known to be involved in olfactory processing [1, 3].
Method: Resting state fMRI data were collected from 15 (age between 20-40 yrs.) normal subjects on a 3T MR system (Achieva 3.0 T TX, Philips Medical Systems, Eindhoven, Netherlands) using an 8-channel phased array coil. The echo planar imaging (EPI) sequence had the following parameters: slice thickness: 4 mm with no gap; matrix size: 64×64; FOV: 192×192mm2; TR/TE: 2000ms/30ms; FA: 90°. Two hundred and thirty (230) resting state data volumes, covering the entire brain with 35 axial slices per volume, were collected from each participant. A high-resolution 3D-T1 weighted structural scan was also acquired for functional overlay with the following parameters: TR / TE / FA = 7600ms / 3400ms / 8°, FOV = 256 mm × 256 mm × 192 mm, slice thickness = 1 mm with no gap between slices, and acquisition time = 7min18 s. Standard fMRI preprocessing steps were performed using SPM12 software. The clustering technique, discussed in detail in R.J. Janssen et al., 2015 [2], is based on an Infinite Gaussian Mixture Model (IGMM) and performs parcellation solely based on POC time courses. In the mixture model, each voxel is assumed to belong to a cluster and voxels within a cluster are assumed to have the same underlying BOLD time course and voxel specific noise structure.
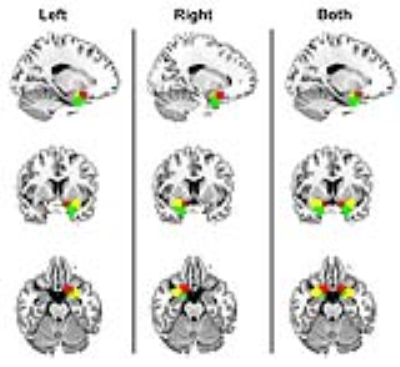
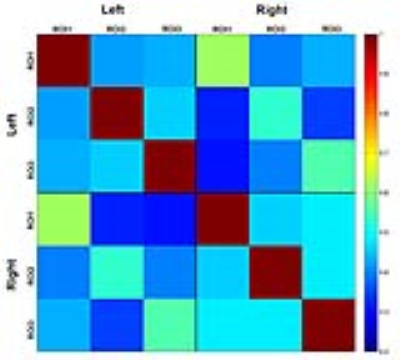
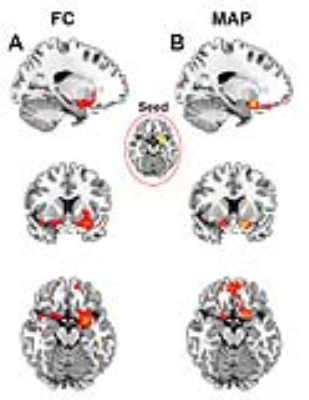
Results: Maximum a posteriori (MAP) estimates of the left POC, right POC, and both the left and right POC parcellations were obtained using concatenated data from 15 study participants (Figure 1). A strong inter-hemispheric correspondence of clusters is evident in the left and right POC parcellations (Figure 1). A similar inter-hemispheric correspondence of clusters is also present in their functional signals as illustrated by the correlation matrix of cluster time courses (Figure 2). Both observations provide clear indication of well-defined functional relationships, including their homologous nature, between POC clusters. The roles of these clusters were further examined using parcellations as seed regions in subsequent functional connectivity (FC) analyses. Figure 3 shows the distinct pattern of FC obtained from one left hemisphere parcellation. Functional connectivity shows that remote regions are not involved in olfactory processing suggesting functional specialization of POC structures.
Discussion: Results of this study suggest that multiple regions within the POC, with clear inter-hemispheric correspondence and functional relevance, can be identified using resting state fMRI data. These parcellations seems to support core and extended FC to orbitofrontal and frontopolar regions outside the POC. Such information is critical in understanding the correspondence between resting state and task-active behaviors of the POC [4, 5]. Finally, tailored parcellations of the olfactory network can be a very useful preclinical tool when investigating Alzheimer’s disease (AD) and Parkinson’s disease (PD), where olfactory deficits usually precede changes in cognition and memory dysfunction [6].
Acknowledgements
This work was supported by the NINDS grant NS060722, the HMC GCRC (NIH M01RR10732), GCRC Construction Grants(C06RR016499), and the Department of Radiology, Pennsylvania State University College of Medicine. The authors report no conflict of interest.References
[1] J. A. Gottfried (2010), ‘Central mechanisms of odor object perception’, Nature, vol 11,pp 628-640.
[2] R.J. Janssen (2015), ‘Probabilistic model-based functional parcellation reveals a robust,fine-grained subdivision of the striatum’, NeuroImage, vol 119,pp 398-405.
[3] P. Karunanayaka, ‘Intrinsic intranasal chemosensory brain networks shown by resting-state functional MRI’, Neuroreport, vol 27, issue 7, pp 527-31.
[4] G. Deco (2011), ‘The dynamical balance of the brain at rest’, Neuroscientist, vol 1, pp 107-23, doi: 10.1177/1073858409354384. Epub 2010 Dec 31.
[5] P. Karunanayaka, ‘Default mode network deactivation during odor-visual association’, HBM, vol 38, issue 3, pp 1125-1139.
[6] M. Vasavada (2015), ‘Olfactory cortex degeneration in Alzheimer's disease and mild cognitive impairment’, J Alzheimers Dis., vol 45, issue 3, pp 947-58.
Figures