3118
Multimodal microstructure imaging: joint T2-relaxometry and diffusometry to estimate myelin, intracellular, extracellular, and cerebrospinal fluid properties1Signal Processing Lab (LTS5), École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Radiology Department, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland, 3Computer Science Department, University of Verona, Verona, Italy
Synopsis
We propose a multimodal joint estimation that aims at exploiting the complementary information of diffusion and multi-echo spin echo data to disentangle the contributions and properties of the main tissue microstructure compartments. We recovered T2, diffusion coefficient, and volume fractions values of myelin, intracellular, extracellular, and cerebrospinal fluid compartments within an ex vivo spinal cord sample by means of diffusometry and relaxometry. A g-ratio map was also calculated.
Introduction
Magnetic Resonance Imaging (MRI) of nervous tissue microstructure is a rather complex task due to the fact that one modality can be sensitive to some tissue properties being almost blind to others. This is the case of multicomponent analysis of multi-echo spin echo data, i.e. T2-relaxometry1, which allows estimating the volume fraction and T2 values of myelin and cerebrospinal fluid (csf), but that hardly allows distinguishing between the contributions of intracellular and extracellular compartments. Similarly, diffusometry1 - the multicomponent analysis of diffusion data - gives information about intracellular, extracellular, and cerebrospinal fluid compartments, but has little sensitivity to myelin due to its characteristic T2, too short compared to typical echo times (TE) used for PGSE2 sequences.
We propose to jointly perform and estimate T2-relaxometry and diffusometry in a synergic manner to disentangle the T2 value, diffusion coefficient, and volume fraction of each compartment separately. To this end, we propose a non-linear multimodal approach. We show results on ex vivo spinal cord data3, collected in a favorable experimental setup where diffusion data is acquired along the direction perpendicular to axons. However, the multimodal approach could be extended to general relaxometry and diffusion MRI acquisitions previous formal modeling. In the following, we present the proposed method, the results, and, our discussion and conclusion.
Methods
The ex vivo spinal cord dataset3 contains a 64x64 voxels slice with a multi-echo spin echo acquisition (32 echoes, $$$ESP/TR=10ms/3s$$$, 8 averages) and a 1-dimensional PGSE acquisition perpendicular to the spinal cord longitudinal direction, from which we selected 50 samples with $$$TE_{diff}=47ms$$$ equally distributed in b-value, $$$b$$$, with maximum gradient strength $$$G_{max}=0.6\,T/m$$$, pulse duration $$$\delta=8ms$$$, and separation $$$\Delta=25ms$$$.
We propose to jointly estimate microstructure parameters from the two modalities, precisely the transverse relaxation time $$$T_2$$$, diffusion coefficient $$$d$$$, and volume fraction $$$f$$$ for the myelin, $$$mye$$$, intracellular, $$$int$$$, extracellular $$$ext$$$, and cerebrospinal fluid $$$csf$$$ compartments:
$$S(TE,b,K) = K \sum_{i\in\{mye,int,ext,csf \} }f_i\, e^{-\frac{TE}{T_2^i}} e^{-bd_i}\,\,\,s.t.\,\,\, \sum_{i\in\{mye,int,ext,csf \} }f_i=1$$
where $$$S$$$ is the description of the T2-weighted diffusion signal, and $$$K$$$ is a constant. To compensate for modality-related instrumental differences, different constants are used: if $$$Y_{d}(b)$$$ and $$$Y_{me}(TE)$$$ are the acquired diffusion and multi-echo signals, then we set $$$K_{me}=\hat{Y}_{me}(0)$$$ for multi-echo and $$$K_{d}\approx K_{me} \frac{\hat{Y}_{d}(0)}{\hat{Y}_{me}(TE_{diff})}$$$ for diffusion. Estimation of constants was performed after non-negative least squares spectroscopy1, which was also used to initialize the non-linear routine minimizing the following joint objective function
$$\frac{ \sum_j^{N_d} [{S}(TE_{diff},b_j,K_d)-Y_d(b_j)]^2}{N_d} + \frac{ \sum_k^{N_{me}} [{S}(TE_k,0,K_{me})-Y_{me}(TE_k)]^2}{N_{me}}$$
$$$N_{d}$$$ and $$$N_{me}$$$ being the number of samples of the related signals.
Results
As the non-linear problem is hard to solve, the optimization was constrained imposing large bounds on parameters, based on visual inspection of non-parametric spectroscopy results. Moreover, due to the low sensitivity of diffusion data to myelin at this echo-time, we neglected its contribution for the first addend of the objective function. Similarly, due to low separability between intra- and extracellular transversal relaxation times with multi-echo data, we approximated $$$T_i=T_e=T_{ie}$$$.
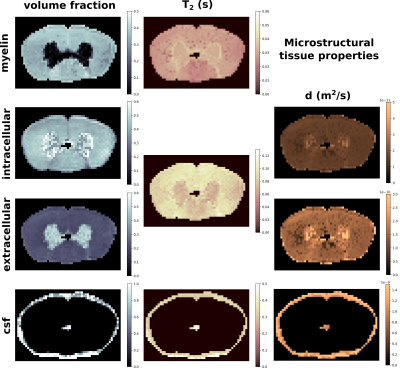
In Fig. 1 we show the estimated quantities for the myelin, intracellular, extracellular, and cerebrospinal fluid compartments, obtained with the proposed joint estimation method. The average values for white matter are $$$f_{mye}=.35$$$, $$$f_{int}=.42$$$, $$$f_{ext}=.22$$$, $$$T_2^{mye}=26ms$$$, $$$T_2^{ie}=87ms$$$, $$$d_{int}=1.6e^{-11}m^2/s$$$, $$$d_{ext}=1.5e^{-10}m^2/s$$$. In the cerebrospinal fluid found in the spinal cord canal the average values are $$$T_2^{csf}=411ms$$$, and $$$d_{csf}=1.08e^{-9}m^2/s$$$.
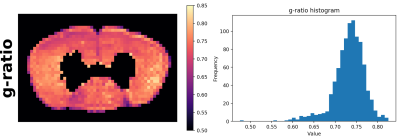
In Fig. 2 we report the g-ratio4 map calculated with results shown in Fig. 1, and the related histogram. The average value is $$$0.73$$$.
Discussion
The proposed multimodal joint estimation aims at exploiting the complementary information of diffusion and multi-echo spin echo data to disentangle the contributions and properties of the main microstructure compartments. Results in Fig. 1 show expected proportions of intracellular, extracellular, and myelin volume fractions. Cerebrospinal fluid is correctly detected in the spinal cord canal, and around the sample. T2 values are correctly ordered and match expected values. Also the diffusion coefficients well distinguish different compartments, which have values approximately separated by one order of magnitude. Finally, the g-ratio map in Fig. 2 reports values perfectly aligned with literature5. Despite the presence of partially non-smooth regions, maps look substantially regular and coherent.
Conclusion
In this work we presented a novel multimodal approach to infer microstructure properties by means of a joint estimation of diffusometry and relaxometry data acquired in an experimental setup. Results are compelling, and their validation by means of histological data is currently ongoing. Nonetheless, the proposed method has the potential to be very useful for microstructure investigation, and it will be further improved, for instance, by adopting a more robust estimation procedure that will possibly allow disentangling additional properties of the tissue microstructure.Acknowledgements
This work is supported by the Swiss National Science Foundation under grant number CRSII5_170873.References
1. Whittall, K.P., MacKay, A.L.: Quantitative interpretation of NMR relaxation data. Journal of Magnetic Resonance, 1989.
2. Stejskal, E.O., Tanner, J.E.: Spin Diffusion Measurements: Spin Echoes in the Presence of a Time Dependent Field Gradient. JCP, 1965.
3. Vuong, M.-T., Duval, T., Cohen-Adad, J., Stikov, N., 2017. On the Precision of Myelin Imaging: Characterizing Ex Vivo Dog Spinal Cord with MRI and Histology, in: Proceedings of the 25th Annual Meeting of ISMRM. p. 3760.
4. Stikov et al.: In vivo histology of the myelin g-ratio with magnetic resonance imaging. NeuroImage, 2015.
Figures