3114
Feasibility of VERDICT-MRI for non-invasive characterisation of rectal cancer microstructure1Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 2University College London Hospital, London, United Kingdom, 3Centre for Medical Imaging, University College London, London, United Kingdom, 4Department of Pathology, University College London Hospital, London, United Kingdom
Synopsis
This work evaluates the feasibility of in-vivo microstructure imaging for rectal cancer using the VERDICT MRI framework. We perform a model comparison to find the form of VERDICT that can describe the rich DW-MRI data. Preliminary results from two subjects show promise for non-invasive clinical rectal cancer
INTRODUCTION
Imaging is fundamental to rectal cancer diagnostic and treatment planning. However, there are limitations in the current MRI techniques for rectal cancer staging, predicting response and prognosis1. The Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours (VERDICT) framework2 could improve currently used imaging techniques via superior staging, prognostication, and evaluation of treatment response. VERDICT uses a rich in-vivo diffusion-weighted MRI (DW-MRI) acquisition with a computational tissue model to estimate tumour parameters such as cell size, vascular and cellular volumes/density, and perfusion, permitting more precise biological phenotyping. VERDICT has shown promise for clinical differentiation of benign and cancerous tissue3, as well as characterisation of specific cancer grades4. The feasibility of VERDICT modelling in rectal cancer has been shown via a preclinical study2, demonstrating its use in differentiation of cancer type and response to treatment. Here we explore the translation of VERDICT to clinical rectal cancer imaging, to obtain non-invasive microstructure-specific measures.METHODS:
MR imaging:
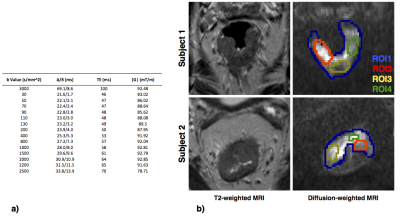
Two male subjects with biopsy-proven rectal cancer were recruited for the study. DW-MRIs were acquired on a 3T-Philips Achieva MRI, using PGSE sequence (EPI read-out) and 1.25x1.25x5mm3 resolution. The acquisition had multiple b-values and diffusion times, summarised in Figure.1a, to support estimation of various multi-compartment models. A high-resolution T2-weighted MRI was acquired for anatomical reference. Motion correction5 was applied to account for tissue movement.
Data analysis
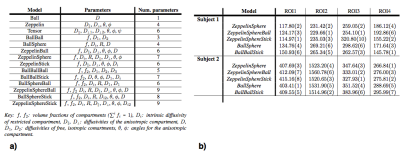
To assess which microstructural models best explain the signal in rectal tissue, we compare 13 plausible compartment models listed in Figure.1b. These models are combinations of: sphere (isotropic diffusion restricted with radius R), stick (diffusion restricted to a single direction), ball (isotropic free diffusion) and zeppelin (anisotropic cylindrically symmetric free diffusion), using terminology in6. We also include the conventional models: ADC (Ball) and IVIM (BallBall). We do model fitting using the non-linear fitting procedure as used in3, with data normalised using the b=0 for each echo time, to account for T2 dependence.
A certified board radiologist drew regions-of-interest (ROIs) to include cancerous tissue from the rectal wall, as shown in Figure.2a. We perform model fitting for the ROIs without fixing any of the model parameters. To find the model which best describes the diffusion signal, we do model comparison using the Bayesian Information Criterion (BIC)7. To demonstrate microstructure parametric maps over the rectal tissue, we do voxel-wise fitting with one of the best-ranked models. To improve fitting stability for these maps we fix the intrinsic diffusivity to $$$1.3\times10^{-9}m^2/s$$$, an indicative value from the ROI analysis8.
RESULTS:
Model comparison
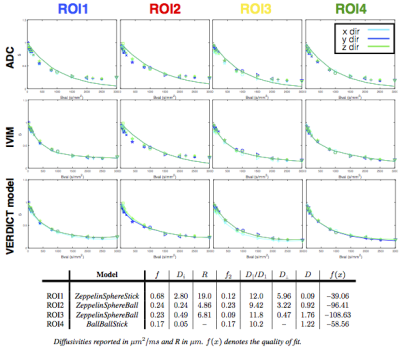
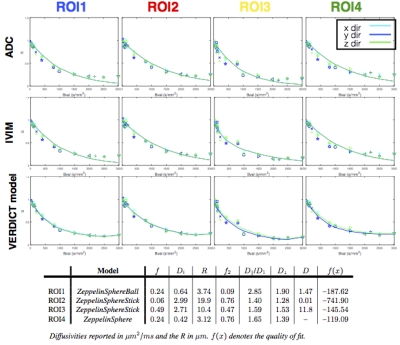
Figures.3&4 show that two- and three-compartment models that include the sphere compartment best fit the data. The simpler ADC and IVIM models fail to capture the signal particularly for higher b-values as expected. Comparison of the fit of the models to the data shows that among two-compartment models, the ZeppelinSphere performs the best, while for three-compartment models the ZeppelinSphereBall and ZeppelinSphereStick perform the best.
To find the simplest model (least number of parameters) that provides the best fit for the data, we rank the models according to BIC, shown in Figure.2b. We see that overall, the two-compartment ZeppelinSphere has the best ranking, with ZeppelinSphereBall a close second.
Parameter maps
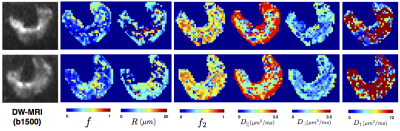
We generate parametric maps using the more general three-compartment model ZeppelinSphereBall that was ranked among the best for both datasets across all ROIs. Figure.5 shows these maps, which include the volume fraction of the restriction compartment ($$$f$$$), intrinsic and vascular diffusivity ($$$D_i$$$, $$$D_1$$$), and the estimated cell dimensions ($$$R$$$). The $$$f$$$ maps show an elevation of the intracellular volume fraction in the cancer tissue and the high $$$D_1$$$ values indicate vascular component.
DISCUSSION AND CONCLUSION
Early results show promise for non-invasive rectal characterisation using VERDICT MRI. The models with sphere compartments provide the best fit to the data, showing that isotropic restriction is very important to characterise the rectal cancer signal. The preference of the Zeppelin and Stick compartments in model selection suggests the presence of anisotropy in the signal, particularly for subject 1. Histopathological results for these subjects will shed more light on how well these findings correlate to the microstructure.
We observe anisotropy even with data acquired with only three gradient orientations, and anisotropic models are favoured from model selection. Future work will add more directions to explore this directionality and whether it is cellular or vascular. Future work will also investigate more complex models, e.g. accounting for T2 of different compartments.
A limitation is that the data acquired suffers from distortions, due to magnetic susceptibility, which is a key difficulty resulting from imaging structures near to tissue-tissue or tissue-air boundaries. Future imaging protocol will aid reduction of such distortions using methods like in9.
Acknowledgements
This work is funded by the EPSRC grant EP/N021967/1 (EP). EP/M020533/1 and EP/N018702/1 support DCA.References
1. Engin, G. & Sharifov, R. Magnetic resonance imaging for diagnosis and neoadjuvant treatment evaluation in locally advanced rectal cancer: A pictorial review. World J. Clin. Oncol. 8, 214–229 (2017).
2. Panagiotaki, E. et al. Noninvasive Quantification of Solid Tumor Microstructure Using VERDICT MRI. Cancer Res. 74, 1902–1912 (2014).
3. Panagiotaki, E. et al. Microstructural Characterization of Normal and Malignant Human Prostate Tissue With Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours Magnetic Resonance Imaging. Invest. Radiol. 50, 218–227 (2015).
4. Johnston, E. et al. Short term repeatability of microstructural (VERDICT) MRI vs. ADC in prostate cancer. in ISMRM (2016).
5. Modat, M., Dagga, P., Clarkson, M. J. & Ourselin, S. NiftyReg: (version 1.3). (2010).
6. Panagiotaki, E. et al. Compartment models of the diffusion MR signal in brain white matter: A taxonomy and comparison. NeuroImage 59, 2241–2254 (2012).
7. Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 6, 461–464 (1978).
8. Li, H., Jiang, X., Xie, J., Gore, J. C. & Xu, J. Impact of transcytolemmal water exchange on estimates of tissue microstructural properties derived from diffusion MRI. Magn. Reson. Med. 77, 2239–2249 (2017).
9. Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870–888 (2003).
Figures