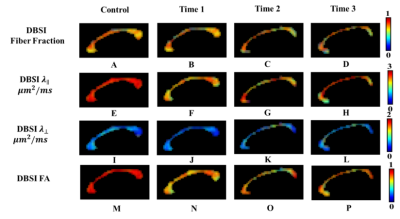3109
Quantification of white matter pathologies during multiple sclerosis disease development1Biomedical Engineering, Washington University in St. Louis, st louis, MO, United States, 2Radiology, Washington University in St. Louis, st louis, MO, United States, 3Neurology, Washington University in St. Louis, st louis, MO, United States, 4The Hope Center for Neurological Disorders, Washington University in St. Louis, St Louis, MO, United States
Synopsis
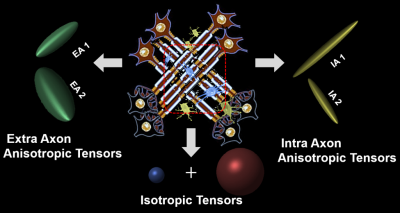
A new diffusion MRI histology (D-Histo) is proposed to model both intra and extra axonal diffusion, in addition to isotropic diffusion. It not only resolves crossing fibers but also quantitatively assess axonal injury, axon loss, demyelination, edema and inflammation. Through the multiple-tensor modelling of diffusion-weighted MRI signals, D-Histo has shown promise to monitor evolving pathologies in normal appearing corpus callosum in multiple sclerosis patient brain.
Introduction
Various diffusion MRI approaches proposed to markers to detect white matter injuries1-3. Complexity of the underlying pathological processes in multiple sclerosis (MS) makes detecting normal appearing white matter injury difficult2,3. We have recently developed diffusion MRI histology (D-Histo) to effectively detect, differentiate and quantify axonal injury and/or loss, demyelination and inflammation individually in MS. In this work we demonstrated in MS patients that D-Histo can quantitatively characterize the distinct pathology patterns in normal appearing corpus callosum. We also demonstrated that D-Histo resolved crossing fibers with intra- and extra-axonal axial diffusivity assessed. Our results suggested that D-Histo hold promise to replace the previously developed DBSI in better characterizing the heterogeneous white matter pathology in MS.Materials and Methods
D-Histo: The new D-Histo models: 1) intra-axonal diffusion 2) surrounding extra-axonal diffusion; and 3) isotropic diffusion of various diffusivities. The normalized diffusion-weighted signal S can be modeled by the equation below: $$$ s_N=\sum^{N_{Aniso}}_{i=1}{f_{i_{intra}}}e^{-\left|\overrightarrow{b_N}\right|\left({\lambda }_{||i\_intra}\right){cos}^2{\theta }_{i,N}}+\sum^{N_{Aniso}}_{i=1}{f_{i_{extra}}}{e^{-\left|\overrightarrow{b_N}\right|{\lambda }_{\bot i\_extra}}e}^{-\left|\overrightarrow{b_N}\right|\left({\lambda }_{||i\_extra}-{\lambda }_{\bot i\_extra}\right){cos}^2{\theta }_{i,N}}+\ \ \int^b_a{f\left(D\right)}e^{-\left|{\overrightarrow{b}}_N\right|.D}d\left(D\right)\ \ $$$
Human subject: Procedures involving human subjects were all approved by the Institutional Review Board of Washington University. Every subject provided informed consent before their participation in the study.
In-Vivo DWI: All subjects underwent diffusion weighted MRI at 3.0T using a multi-b value diffusion weighting scheme (Trio; Siemens, Erlangen, Germany). Diffusion-weighted images (DWIs) were collected with a 99-direction multi-b-value diffusion scheme using a single-shot spin-echo echo planar imaging sequence with the following key parameters: voxel size = 2×2×2 mm3; Maximum b-value = 1500 s/mm2; acquisition time = 15 minutes.
Data analysis:DBSI and D-Histo were computed using the in-house software developed using Matlab.
Results and Discussion
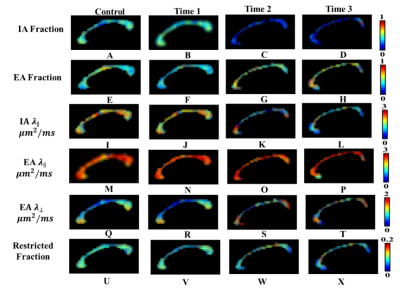
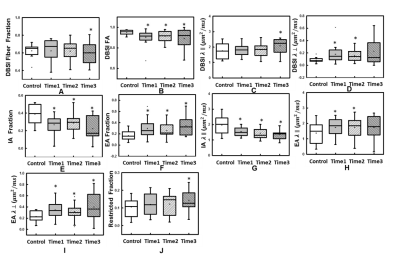
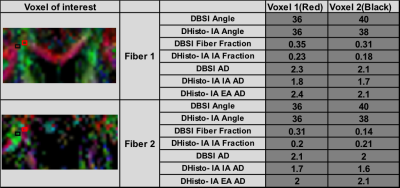
Color-coded DBSI maps of the corpus callosum (CC) region show white matter tract lesions may be detected in MS patients. DBSI AD (axial diffusivities) (Fig. 2F) decreased at time 1 indicating axonal injury in CC. However, a slight increase of DBSI AD (Fig. 2H) at time 3 was observed. DBSI RD increased at time 2 may reflect edema or demyelination (Fig. 2-K). Color-coded DHisto maps of CC suggested axon loss, i.e., intra-axonal (IA) fraction decreased (Fig. 3C); edema, i.e., extra-axonal (EA) fraction increased (Fig. 3G); axonal injury (IA AD decreased, Fig. 3K); demyelination (EA RD increased, Fig. 3S) at time 2. Significantly decreased IA fraction derived from D-Histo was seen in MS patient at time 1 compared to the control group (Fig. 4E). The D-Histo IA AD (Fig. 4G) was significantly decreased at time 1 indicates axonal injury in MS. Demyelination at the time 1 was evidenced by the increased EA RD. The difference of EA fraction between control and MS patient from time 1 was statistically significant indicating edema in CC. To test the ability of D-Histo to resolve crossing fibers with accurate axial and radial diffusivities assessed, DBSI and D-Histo results of the crossing CC and corona radiate of healthy control were compared. D-Histo shows comparable accuracy in resolving crossing fibers as in DBSI.Conclusion
We proposed a novel D-Histo to model diffusion weighted signals resolving crossing fibers and intra-extra-axonal compartments to improve the accuracy of assessing axonal injury and loss. In contrast to other diffusion MRI models4,5,6,7, D-Histo is likely to be less dependent on diffusion time and b-values used in data acquisition. D-Histo results from MS and control subjects herein support the utility of this newly developed diffusion MRI model to more accurately reflect axonal and myelin integrity while estimating the extent of inflammation in MS patient.Acknowledgements
This work was supported in part by NIH R01-NS047592, P01-NS059560, U01-EY025500, National Multiple Sclerosis Society (NMSS) RG 4549A4/1, RG-1507-05315.References
1. Yaniv Assaf, Peter J. Basser. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. NeuroImage 2005; 27:48 – 58
2. Benoit Scherrer, Armin Schwartzman, Maxime Taquet et al. Characterizing Brain Tissue by Assessment of the Distribution of Anisotropic Microstructural Environments in Diffusion-Compartment Imaging (DIAMOND). Magnetic Resonance in Medicine 2016; 76:963–977
3. Enrico Kaden, Nathaniel D. Kelm, Robert P. Carson et al. Multi-compartment microscopic diffusion imaging. NeuroImage 2016; 139: 346–359
4. Wang Y, Wang Q, Song SK et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011; 134: 3590–601.
5. Jian B, Vemuri BC, Ozarslan E et al. A novel tensor distribution model for the diffusion-weighted MR signal. Neuroimage 2007; 37:164–176.
6. Hess CP, Mukherjee P, Han ET et al. Q-ball reconstruction of multimodal fiber orientations using the spherical harmonic basis. Magn Reson Med 2006; 56:104–117.
7. Tournier JD, Calamante F, Gadian DG et al. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 2004; 23:1176–1185.
Figures