3096
Age-effects on cortical tissue diffusivity1Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3MGH/HST Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
Synopsis
Mean diffusivity (MD) is known to increase with age in the white matter (WM), serving as a measure of age-related WM degeneration. However, age-effects on MD with age have scarcely been studied in the gray matter. Here we examine age-effects on MD across the cerebral cortex. MD is shown to correlate more strongly with age than cortical thickness measurements derived from anatomical MRI. Cortical regions showing the largest MD age-effects include the insula and anterior cingulate, suggesting greatest neurodegeneration in these regions. This work suggests that MD may be used as a sensitive measure of aging in the cerebral cortex.
Introduction
Mean diffusivity (MD) is a metric derived from diffusion tensor imaging (DTI). MD has been shown to be a sensitive measure of healthy adult aging in the white matter (WM).1 Increased MD with age in the WM likely reflects age-related WM degeneration and has been shown to correlate with cognitive decline.1 MD has also been shown to correlate with cognitive decline in the gray matter (GM) cortex of older adults.2 However, age-effects on MD in the GM cortex have scarcely been studied, other than a few reports that MD increases globally with age in the cortex.3-5 The cortex is known to shrink with age.6 Degenerated spaces in the aging cortex are filled by free water, and this can confound age-effects on cortical thickness measurements derived from structural MRI. Since MD directly measures water diffusion, MD could potentially serve as a more sensitive measure of cortical aging. In this work, we examine age-effects on MD across the cerebral cortex and compare with age-effects on MRI-derived cortical thickness measurements.Methods
101 healthy subjects aged 39.1 to 86.1 years (60.6 + 11.5) were imaged on a 3T Siemens Magnetom Trio system with a 12-channel head coil. Diffusion MRI (dMRI) with 60 directions was performed at b=700mm2/s following 10 b=0 volumes. Other parameters were TE=83ms, TR=7.98s, FOV=25.6cm, 2mm3 resolution, 70 slices. All dMRI data sets were corrected for motion and eddy currents using eddy_correct7 in FSL,8 and masked using FSL's Brain Extraction Toolbox (bet).9 DTI was fitted to obtain DTI parameter maps. Anatomical data was obtained for all subjects via multi-echo MPRAGE at TE=1.64ms, TR=2530ms, FOV=25.6cm, 1mm3 resolution, 176 slices. Cortical reconstruction was performed and cortical thickness was calculated using FreeSurfer.10 DTI parameter maps were projected onto cortical surfaces via FreeSurfer’s mri_vol2surf (projfrac=0.5) and registered to a standard (fsaverage) surface. Effect size (change in parameter per year) was fitted using FreeSurfer’s mri_glmfit, thresholded at p<0.05 and corrected for multiple comparisons (cluster-wise correction via FreeSurfer’s mri_glmfit-sim).Results
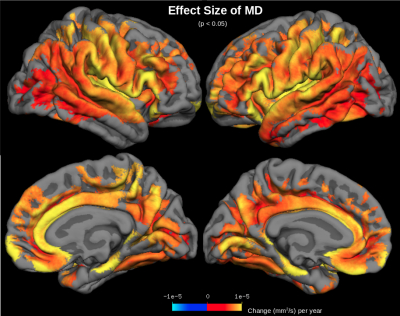
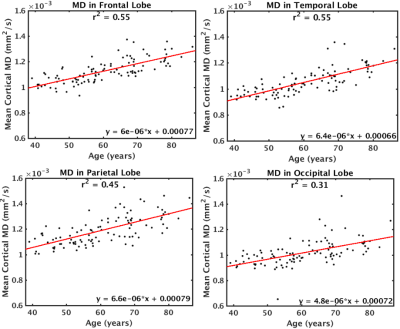
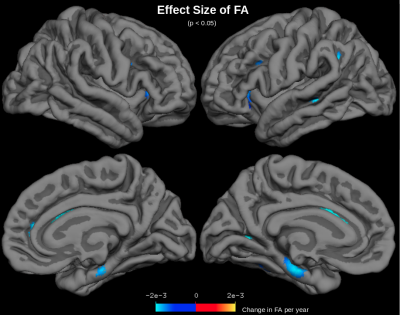
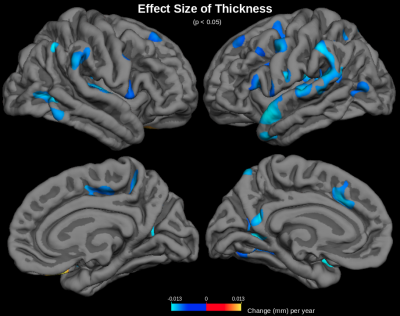
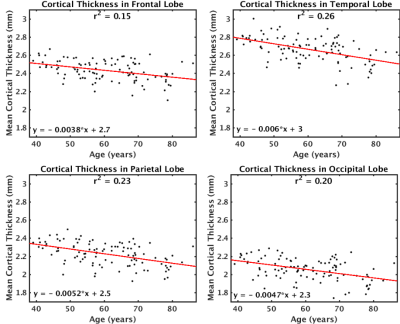
MD was found to increase with age across the cerebral cortex (Figure 1). The largest increases in MD with age were found in the insula, anterior cingulate, inferior frontal gyrus (particularly the pars opercularis) and parts of the parahippocampal gyrus. Correlations of MD with age were strongest in the frontal and temporal lobes (r2=0.55) and weakest in the occipital lobe (r2=0.31) (Figure 2). The parietal lobe showed the largest effect size of MD increase with age (an additional 6.6x10-6 mm2/s per year), and the occipital lobe showed the smallest effect size (an additional 4.8x10-6 mm2/s per year). Conversely, another DTI parameter, fractional anisotropy (FA), exhibited little age-effects in the cortex (Figure 3). Only the temporal lobe displayed a moderate (negative) association of FA and age (r2=0.21, ranging from FA=0.22 to FA=0.20 between age 40-90). Cortical thickness was also found to decreases with age, although this effect was much less prevalent in the cerebral cortex compared with age-effects on MD (Figure 4 & 5).Discussion
MD appears to be a sensitive measure of the aging cerebral cortex, displaying increases with age across the cortex. Increased MD with age likely reflects neurodegeneration-associated increases in interstitial water and increases in partial-volume effects with cerebrospinal fluid (CSF) at older ages. Areas of largest MD increase with age suggest areas of most significant age-related degeneration. Fractional anisotropy (FA) is another DTI parameter that measures aging in the WM, but FA shows little age-effects across the cerebral cortex, presumably because gray matter is generally less anisotropic than WM. Moreover, age-effects on cortical MD are much more prominent across the cerebral cortex than age-effects on MRI-derived cortical thickness measurements. This is likely because free water occupies degenerated spaces at older ages, confounding cortical thickness measurements. MD is presumably a more sensitive measure of aging because it directly measures increased free water diffusion at older ages.Conclusions
In this study, it was found that mean diffusivity (MD) derived from DTI serves as a sensitive measure of aging in the cerebral cortex. MD appears to detect increased free water diffusion that results from neurodegeneration and which MRI-derived cortical thickness measures cannot detect. This work suggests that MD may be used as a measure of cortical aging in future DTI studies.Acknowledgements
We thank the Canadian Institutes of Health Research (CIHR) and the National Institutes of Health (NIH) for financial support.References
1. Madden EJ, et al (2012) Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta 1822(3):386-400
2. Kantarci K, et al (2011) Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 77(1):26-34
3. Abe O et al (2008) Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging 29:102-116
4. Jeon T et al (2012) Regional changes of cortical mean diffusivities with aging after correction of partial volume effects. Neuroimage 62:1705-1716
5. Salminen LE (2016) Regional age differences in gray matter diffusivity among healthy older adults. Brain Imaging and Behavior 10:203-2011
6. Peters R (2006) Ageing and the brain. Postgrad Med J 82(964):84-88
7. Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration ofbrain images. Med Image Anal 5(2):143-156
8. Smith SM, et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208-219
9. Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143-155
10. Fischl B (2012) FreeSurfer. Neuroimage 62(2):774-781
Figures