3095
Retrospective Reduction of Systematic Differences Across Scanner Changes by Accounting for Noise Floor Effects in Diffusion Tensor Imaging1Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 2Life Science MRI Facility, Purdue University, West Lafayette, IN, United States, 3Keller Center for Imaging Innovation, Barrow Neurological Institute, Phoenix, AZ, United States, 4Neurological Institute, The Cleveland Clinic, Cleveland, OH, United States
Synopsis
Scanner upgrades are a persistent but important problem when conducting MRI research studies. Systematic differences introduced by a scanner upgrade can have undesirable effects on the conclusions of a study. Quantitative tissue microstructure measurements by diffuson tensor imaging (DTI) can be affected by systematic differences in noise floor effects. Noise floor effects are due to rectification of signal by magnitude reconstruction than can, in turn, bias microstructure measurements. A retrospective correction that accounts for noise statistics is proposed to limit systematic differences in DTI measurements across scanner upgrades. A practical measure, signal to noise floor ratio (SNFR) is proposed to determine the conditions under which the retrospective correction works effectively.
Introduction
Changes in scanner configuration can negatively impact quantitative MRI studies by introducing systematic differences between measurements that are due to the instrument, not biology. Noise floor rectification is a potential source of systematic differences in diffusion tensor imaging (DTI)1. The purpose of this study is to determine if retrospective correction for noise floor effects can reduce systematic differences and improve reproducibility across major scanner changes.Methods
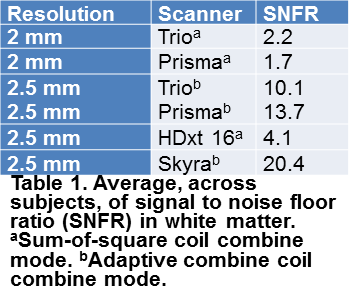
Three sites implemented an upgrade from a Siemens TIM Trio to a Siemens Prisma (Siemens Medical Solutions, Erlangen, Germany) and the other replaced a GE Signa HDxt 16x (GE Medical Systems, Waukesha, USA) with a Siemens Skyra. At one site, 6 subjects were scanned at 2 mm isotropic resolution (71 b=1000sec/mm2, 8 b=0) on both Trio and Prisma platforms under a protocol approved by the local IRB. All sites implemented an acquisition at 2.5 mm isotropic resolution that was matched across platforms (64 b=700sec/mm2, 8 b=0), with a notable difference being use of adaptive coil combination (AC)2 on Siemens and sum of squares coil combination (SOS)3 on the GE, and 14 subjects were scanned under a protocol approved by a central IRB implemented within a multicenter trial4. The diffusion tensor was calculated in each voxel by standard log-linear least squares (LLSQ)5. To account for noise floor effects, the tensor was also fit by a maximum likelihood estimation (MLE) method that takes into account non-central chi signal statistics6. Standard measures of tissue microstructure (fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD)7) were compared across the scanner upgrades using the methodology described by Schwarz et al.8 A simple metric, signal to noise floor ratio (SNFR), was constructed to determine the conditions under which a correction is helpful. SNFR is the ratio of the minimum value in each voxel among diffusion-weighted signals (that most likely to be affected by noise floor bias) and an estimate of the noise floor level (the first moment of the noncentral-chi distribution9 estimated during the MLE fit).Results
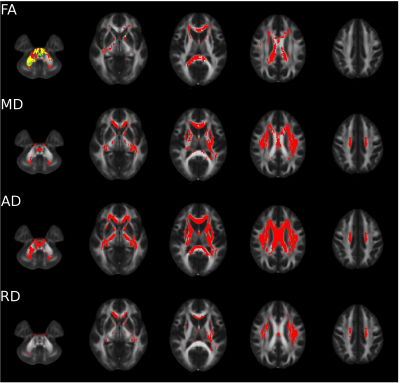
At 2mm resolution,
there are statistically significant systematic differences throughout white
matter, as indicated by red voxels in figure 1. However, after accounting for
noise floor effects with MLE, systematic differences are less extensive, as
indicated by green voxels. After accounting for the noise floor with MLE, none of the voxels in the MD, AD and RD showed
statistically significant systematic differences. At 2.5 resolution for the
Trio/Prisma upgrades, only 0.05% of white matter voxels had significant systematic
differences in FA maps derived from LLSQ. In contrast with the 2mm data,
accounting for noise floor effects by MLE exacerbated systematic differences in
FA maps, with 4% of white matter voxels having significant differences. For MD,
LD and TD, no significant systematic differences were found for either LLSQ or
MLE at 2.5mm resolution. For the HDxt 16 / Skyra change, no statistically
significant systematic differences were found for any of the microstructure
parameters for LLSQ or MLE.
SNFR in white
matter was higher with larger voxels and was higher with AC than with SOS (figure 2). The higher SNR associated with larger voxels and the lower noise floor
associated with adaptive coil combinaton underlies the differences in SNFR.Conclusion
This work demonstrates that retrospective correction for noise floor effects can ameliorate systematic differences and enhance reproducibility in DTI measurements across scanner upgrades. The proposed correction should be used judiciously, as benefit is found primarily with sufficiently low signal as compared to the noise floor (SNFR < 3). This and further corrections are expected to improve statistical power of DTI studies by limiting instrument-related systematic noise.Acknowledgements
This work was supported by the National Institutes of Health [grant number 1U01NS082329-01A1] and the National Multiple Sclerosis Society [grant number RG4931A1/1]. We gratefully acknowledge support from the SPRINT-MS network (https://www.neuronext.org/nn102-sprint-ms) and from John Kirsch and Johan Tondeur of Siemens Healthineers.References
1. Jones, D. K. & Basser, P. J. "Squashing peanuts and smashing pumpkins": how noise distorts diffusion-weighted MR data. Magn Reson Med 2004; 52(5):979-993.
2. Walsh, D. O., Gmitro, A. F. & Marcellin, M. W. Adaptive reconstruction of phased array MR imagery. Magn Reson Med 2000; 43(5):682-690.
3. Roemer, P. B., Edelstein, W. A., Hayes, C. E., Souza, S. P. & Mueller, O. M. The Nmr Phased-Array. Magnetic Resonance in Medicine 1990; 16(2):192-225.
4. Fox, R. J. et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016; 50(166-177.
5. Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994; 66(1):259-267.
6. Sakaie, K. & Lowe, M. Retrospective correction of bias in diffusion tensor imaging arising from coil combination mode. Magn Reson Imaging 2017; 37(203-208.
7. Basser, P. J. & Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111(3):209-219.
8. Schwarz, C. G. et al. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage 2014; 94(65-78. 9. Constantinides, C. D., Atalar, E. & McVeigh, E. R. Signal-to-noise measurements in magnitude images from NMR phased arrays. Magn Reson Med 1997; 38(5):852-857.
9. Constantinides, C. D., Atalar, E. & McVeigh, E. R. Signal-to-noise measurements in magnitude images from NMR phased arrays. Magn Reson Med 1997; 38(5):852-857.
Figures