3085
Hippocampal Subfield-specific Tractography in Epilepsy Patients at 7 Tesla1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
This is the first investigation to use diffusion tensor imaging at 7 Tesla to quantify changes in the structural connectivity of individual hippocampal subfields in epilepsy patients. Diffusion imaging and automated hippocampal subfield segmentation were performed on 19 epilepsy patients and 10 healthy controls. We found that hippocampal volumes are reduced bilaterally in epilepsy patients compared with controls. Connectivity in the left fimbria and right hippocampal-amygdaloid transition area is significantly reduced in epilepsy patients compared with controls. These findings suggest that connectivity of hippocampal subfields are independently affected in epilepsy patients.
Introduction
Medial temporal lobe (MTL) epilepsy is the most common type of epilepsy, affecting approximately 0.1% of the population [1]. Hippocampi, which reside in the MTL, are often involved in seizure onset [1,2]. Since hippocampi are composed of functionally distinct subfields, measures of subfield-specific changes in epilepsy patients may help in understanding epileptogenesis [2]. Previous studies have found volumetric reductions in specific subfields in epileptic patients, as well as global hippocampal atrophy [2]. In addition, abnormal structural connectivity in hippocampi and other limbic structures such as the amygdala has been reported in epilepsy patients [3]. It is thought that seizure activity damages axonal bundles, causing a reduction of fiber density. However, conclusive connectomic changes in epilepsy have not been identified, especially with regard to hippocampal subfields. The current study employs ultra-high field (7 Tesla) MRI to delineate hippocampal subfields and achieve subfield-specific tractography. Here we report the first application of subfield-specific measures in epilepsy patients and controls.Methods
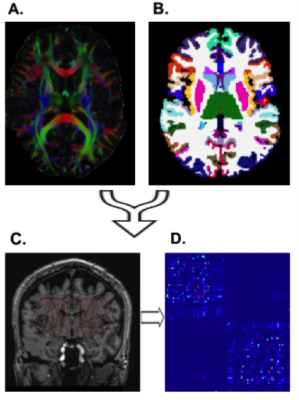
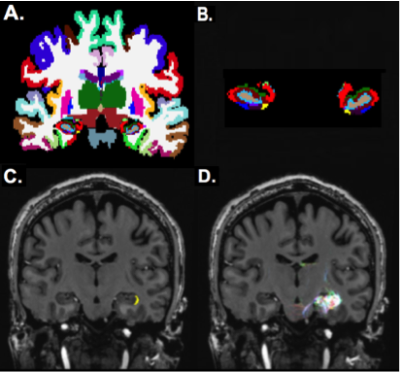
Nineteen epilepsy patients and 9 healthy controls were scanned under an approved IRB-approved protocol using a 7T whole body scanner (Siemens, Magnetom). The MRI scan included a T1-weighted MP2RAGE sequence (0.7 mm isotropic resolution) and high-angular-resolved diffusion-weighted dMRI (1.05 mm isotropic resolution, 68 directions). Diffusion-weighted images were corrected for distortions and co-registered with T1-weighted images. Wholebrain tractography (Figure 1A) was performed using spherical deconvolution and the iFOD2 [4] algorithm and SIFT in MRTRIX3 to identify 10,000,000 fibers. The diffusion data was co-registered with the Freesurfer segmentation (Figure 1B) yielding global anatomical network construction (Figure 1C) and enabling structural connectivity matrices (Figure 1D). In addition, Freesurfer (http://freesurfer.net/) was used for wholebrain segmentation on T1-weighted images (using the default recon-all function) and for hippocampal subfield segmentation [5] using T1 and T2 images (Figure 2A,B). Subfield segmentations were used as masks to faciliate subfield-specific tractography (Figure 2C,D). The degree of each hippocampal subfield was calculated by summing the total number of streamlines connecting that subfield to the rest of the nodes in the structural matrix to generate a measure of total connectivity. Significance of subfield degrees was determined with a two-tailed t-test.Results
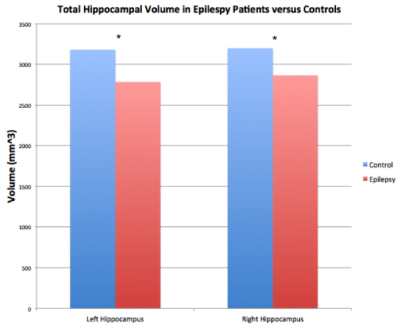
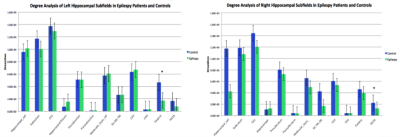
Volumetric analysis (Figure 3) revealed that the left hippocampi of epilepsy patients (Mean = 2783.10 mm3 $$$\pm$$$ 413.27 mm3) were significantly smaller than those of controls (Mean = 3180.51 mm3 $$$\pm$$$ 278.12 mm3). The right hippocampi of epilepsy patients (Mean = 2864.04 mm3 $$$\pm$$$ 274.84 mm3) were also significantly reduced in volume compared to those of controls (Mean = 3197.96 mm3 $$$\pm$$$ 326.82 mm3). This reached significance in both hemispheres (left: p = 0.015; right: p = 0.009). Degree analysis among the hippocampal subfields (Figure 4) revealed significantly reduced streamline counts in the left fimbria in epilepsy patients (Mean = 1730 $$$\pm$$$ 1280) compared with controls (Mean = 4660 $$$\pm$$$ 3430), p = 0.044. The right hippocampal-amygdaloid transition area was also significantly reduced in degree in epilepsy patients (Mean = 1260 $$$\pm$$$ 699) compared to controls (Mean = 2210 $$$\pm$$$ 1290), p = 0.017.Discussion
These results are consistent with previous reports of global reduction in hippocampal volume in epilepsy patients [2]. This study also reveals subfield-specific alterations in connectivity in the hippocampi of epilepsy patients. We found reduced degree of subfield connectivity in the left fimbria and right HATA, which concurs with prior reports of reduced connectivity of the whole hippocampus in epilepsy patients [3]. Reduced degree in these subfields could be associated with axonal damage as a result of electrical insult [3]. Since the fimbria is the major output of the hippocampus, it plays a role in seizure propagation [6]. Indeed, animal studies have found fractional anisotropy is decreased in the fimbria after seizure induction [7]. This finding has also been observed in MTL epilepsy patients, suggesting that the fimbria may be susceptible to damage as a result of seizures [7]. The HATA is a transitional region, forming a bridge between the hippocampus and amygdala [8]. Our results suggest that amygdalo-hippocampal connectivity may be compromised in epilepsy patients. To our knowledge, this is the first study to quantify subfield-specific connectivity in the hippocampi of epilepsy patients. In the future, we plan to further verify and characterize these preliminary findings by expanding our sample size.Acknowledgements
Rafael O’Halloran
NIH R00 NS070821
NIH R01 MH109544
Icahn School of Medicine Capital Campaign
Translational and Molecular Imaging Institute
References
1. Bernasconi, N., Bernasconi, A., Caramanos, Z., Antel, SB., Andermann, F., Arnold, DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain, Volume 126, Issue 2, 1 February 2003, Pages 462–469
2. Mueller, S., Laxer, K., Barakos, J., Cheong, I., Garcia, P., Weiner, M. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-Resolution MRI at 4 Tesla: Preliminary results. Epilepsia, vol. 50, no. 6, 2009, pp. 1474–1483
3. Bonilha, L., Nesland, T., Martz, G. U., Joseph, J. E., Spampinato, M. V., Edwards, J. C., & Tabesh, A. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. Journal of Neurology, Neurosurgery & Psychiatry, 2012. 83(9), 903-909.
4. Tournier J, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In: Proc. 18th Annual Meeting of the Intl. Soc. Mag. Reson. Med. ISMRM, 2010. P.1670.
5. Iglesias, J.E., Augustinack, J.C., Nguyen, K., Player, C.M., Player, A., Wright, M., Roy, N., Frosch, M.P., McKee, A.C., Wald, L.L., Fischl, B., and Van Leemput, K.A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage, 115, July 2015, 117-137.
6. Parekh, Mansi B., et al. Early MR diffusion and relaxation changes in the parahippocampal gyrus precede the onset of spontaneous seizures in an animal model of chronic limbic epilepsy. Experimental Neurology, vol. 224, no. 1, 2010, pp. 258–270.
7. Concha, Luis, et al. Bilateral White Matter Diffusion Changes Persist after Epilepsy Surgery. Epilepsia, vol. 48, no. 5, 2007, pp. 931–940.
8. Fudge, J.l., et al. Revisiting the hippocampal–amygdala pathway in primates: Association with immature-Appearing neurons. Neuroscience, vol. 212, 2012, pp. 104–119.
Figures