3084
Interferon-alpha induced changes in NODDI predispose to the development of fatigue1Neuroscience and imaging, Brighton and Sussex Medical School, Brighton, United Kingdom
Synopsis
Here we use NODDI modeling of multi-shell diffusion MRI to investigate whether changes in orientation-dispersion index (ODI) or intracellular volume fraction (Vic) can predict the later emergence of IFN-α-induced fatigue. Eighteen patients initiating IFN-α based treatment for hepatitis-C underwent diffusion MRI and blood sampling at baseline and 4 hours after their first IFN-α injection. They were then followed up with regular psychological assessments for 12 weeks of treatment. IFN-α injection stimulated an acute inflammatory cytokine response and evoked acute fatigue that peaked between 4 and 12 weeks of treatment. Within the brain, IFN-α induced an acute increase in intracellular volume fraction in patients that experienced a simultaneous increase in IFN-α induced fatigue but not patients that did not. Acute changes in striatal microstructure additionally predicted the continued development of fatigue but not mood symptoms 4 and 8 weeks later into treatment. Our findings highlight the value of NODDI as a potential in vivo biomarker of the central effects of peripheral inflammation. We highlight the exquisite sensitivity of the striatum to IFN-α and further implicate striatal perturbation in IFN-α-induced fatigue.
Aim and audience
This aim of this work was to identify sensitive biomarkers for the central effects of peripheral inflammation using advanced quantitative MR imaging techniques and to support the interpretation of findings from a quantitative magnetization transfer imaging study performed on the same cohort. This work is especially relevant to researchers and clinicians with an interest in fatigue and inflammation.
Introduction
Interferon-alpha (IFN-α) is a type I interferon that promotes broad antiviral immune responses and is used in the treatment of Hepatitis-C infection. Despite good clinical efficacy, the actions of IFN-α on the brain frequently result in highly disabling effects on fatigue, motivation, mood, and cognitive impairments1. Symptoms typically emerge within hours of the first IFN-α injection suggesting that IFN-α can rapidly engage immune-brain communicatory pathways1,2.
Quantitative magnetization transfer (MT) has shown that IFN-α results in rapid (within 4 hours) changes in the striatum occur, with increased MT rate from free (water) to molecular-bound protons (kf) and a complementary reduction in free water spin-spin relaxation (T2f)2,3. Striatal changes also correlated with between-subject sensitivity to IFN-α-induced motivational change and/or subjective fatigue. Although these qMT metrics are sensitive markers for subtle microstructural change, they are not specific and are difficult to attribute to particular biological processes. Further characterization is possible with the aid of diffusion weighted MRI.
Here we use Neurite Orientation Dispersion and Density Imaging (NODDI)4 to probe three distinct diffusion regimes: unrestricted (isotropic diffusion), hindered diffusion (Gaussian displacement pattern) and restricted diffusion (non-Gaussian displacement pattern). These broadly correspond to the following microstructural environments: CSF, the extra-cellular space and intracellular spaces, respectively. The NODDI model estimates the size of the isotropic diffusion space Viso, the size of the intracellular space within tissue Vic and the neurite orientation dispersion index ODI.
Materials and methods
Participants: Eighteen hepatitis-C patients (mean age=47 years, range=18-64 years, SD=11 years, 13 male) who were selected to receive pegylated IFN-α based therapy.
MRI: Siemens 1.5 T Avanto (Erlangen, Germany) use to acquire NODDI data with single-shot, twice-refocused pulse gradient spin-echo echo planar imaging: voxel size 2.5×2.5×2.5mm3, 60 axial slices, matrix size 96×96, field-of-view 240×240mm2, TR/TE=8400/99ms, b-values = 300, 800 and 2400s/mm2 with 8, 30 and 60 non-colinear directions, respectively. Eleven b≈0 images were acquired. Total acquisition time=17min. Two MR scans were performed: baseline and 4 hours post-treatment with IFN-α.
Fatigue and Depression: Symptoms were evaluated at baseline, at 4 hours following treatment and at weeks 4, 8, and 12 of IFN-α based therapy. Fatigue was measured using a visual analogue scale (fVAS) where participants marked their degree of fatigue on a 10-cm scale. Depression was measured with the Hamilton Depression Rating Scale (HAMD) respectively.
Analysis: FMRIB software library (FSL, version 5.0.7) was used for movement and eddy-current correction. The data was fitted to the NODDI model with the toolbox provided for Matlab5. SPM12 was used for a voxel-wise statistical comparison of normalized parameter maps. We defined four a priori ROIs for analyzing the effects of IFN-α: striatum (left and right) and insula (left and right) based on previous findings2,3.
Results
While there was no main effect of IFN-α within striatal or insula ROIs for the NODDI metrics, there was a significant positive correlation between acute changes in Vic and fatigue in (left) striatal and, to a lesser extent (left) insula ROIs (Table 1), with modestly-large effect size (R2=0.52 and R2=0.46 respectively). Acute changes in Vic in left striatum additionally predicted the magnitude of fatigue experienced 4 and 8 weeks later. In contrast, ODI did not significantly correlate with fatigue at any time-point, suggesting that IFN-α rapidly affects the size of the water spaces within the striatum but does not result in a significant reorganization of the brain microstructure. In keeping with MT findings, no significant association exists between changes in NODDI metrics and mood. Whole-brain analysis confirmed the significant positive correlation between left striatum increases in Vic and development of fatigue at 4 hours post-injection (Figure 1). A number of additional clusters exhibited a similar positive correlation with fatigue (Table 2).
These data suggest that changes in intracellular water play a role in the emergence of IFN-α-induced fatigue. The striatum is reinforced as a key brain area targeted by IFN with implications for impaired motivation. As with previous qMT findings, the absence of a link between changes in NODDI metrics and mood further strengthens the notion that distinct mechanisms underpin the action of IFN-α on fatigue and depressive symptoms.
Conclusions
Our findings highlight the value of NODDI as a potential in vivo biomarker of the central effects of peripheral inflammation. We highlight the exquisite sensitivity of the striatum to IFN-α and further implicate striatal perturbation in IFN-α-induced fatigue.Acknowledgements
This research was funded by a Wellcome Trust Fellowship awarded to NAH (Grant Number: 093881/Z/10/Z). We thank Majella Keller, Alexandra File, and Catherine Wood for their contribution to the recruitment of patients.References
1. Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH (2002). Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology, 26:643-652
2. Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, Harrison NA (2016). Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biol Psychiatry 79:320-328
3. Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, Cercignani M (2015). Quantitative magnetization transfer imaging as a biomarker for effects of systemic inflammation on the brain. Biol Psychiatry, 78:49-57
4. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012). NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61: 1000-1016
5. The NODDI toolbox: http://mig.cs.ucl.ac.uk/mig/mig/index.php/?n=Tutorial.NODDImatlab/
Figures
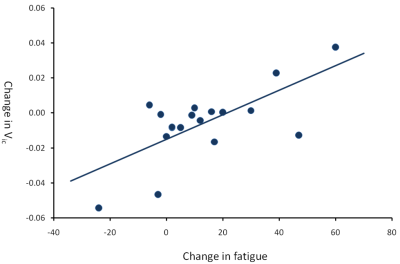
Figure 1 Plot of the correlation between acute change in Vic and acute change in fatigue in the left striatum (R=0.69, p=0.001).
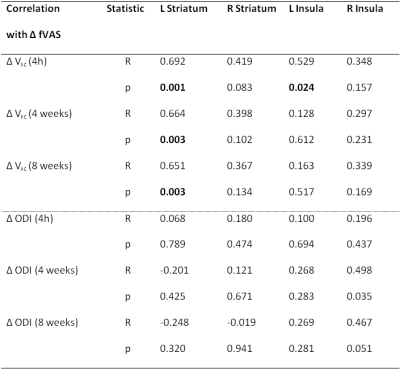
Table 1 Pearson correlation coefficient (R) and statistical significance (p) of correlations between acute change in NODDI metrics (baseline to 4 hours post first IFN-α injection) with change in fatigue at 4 hours (acute), 4 weeks, and 8 weeks post-onset of IFN-α treatment.
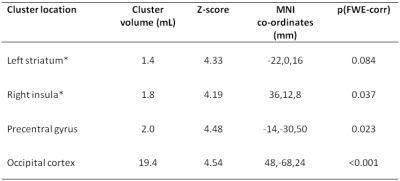
Table 2 Brain areas with positive correlation of acute change in Vic with acute fatigue. * indicates a priori regions of interest. FWE-corr=cluster wise family-wise error-corrected.