3079
DTI Assessment of Regional White Matter Changes in the Cervical and Thoracic Spinal Cord in Pediatric Subjects1Radiology, Thomas Jefferson University, Philadelphia, PA, United States, 2Occupational Therapy, Thomas Jefferson University, Philadelphia, PA, United States, 3Neurosurgery, Thomas Jefferson University, Philadelphia, PA, United States, 4Radiology, Johns Hopkins School of Medicine, Baltimore, MD, United States
Synopsis
Synopsis: Prior adult studies have shown that DTI allows for noninvasive assessment of the severity of spinal cord injury (SCI). The aim of this study was to determine whether DTI at sites cephalad and caudal to the injury provides measures of injury severity in pediatric subjects with chronic SCI and compared these data with normative DTI data of typically developing subjects. ROIs were drawn on whole cord and spinal cord white matter (WM) areas: ventral, dorsal, and both right and left lateral regions along the entire cervical and thoracic SC. For each SCI subject, DTI parameters for each WM region were measured at the levels cephalad and caudal relative to MR injury. We demonstrated changes in FA and AD in WM regions at levels both cephalad and caudal to the injury site. This suggests that FA and AD has the potential to be sensitive marker of true extent of cord injury and might be useful in detecting remote injuries.
Introduction
Introduction: Measurements of water diffusion within the spinal cord (SC) after an injury, including in regions distant from the injury site, may provide valuable insight into the severity of injury. Prior adult studies have shown that DTI allows for noninvasive assessment of the severity of spinal cord injury (SCI)1,2,3. During chronic SCI, the extensive longitudinal spreading of the lesions creates changes in the SC morphology. Changes in the diffusivity associated with these structural changes makes it possible to identify the rostral and caudal extent of the lesion using DTI. The aim of this study was to determine whether DTI at sites cephalad and caudal to the injury provides measures of injury severity in pediatric subjects with chronic SCI. We compared these data with the normative DTI data of the typically developing (TD) pediatric subjects.Methods
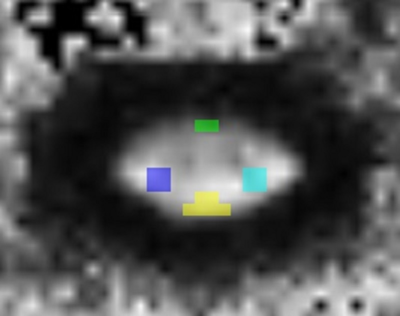
Methods: A total of 10 TD (mean age, 12.0±2.93 years) without evidence of SCI or pathology and 9 subjects with chronic SCI (mean age, 12.48±3.17 years) were included in the study. SCI subjects underwent a neurological evaluation based on ISNCSCI and consisted of AIS A (n=6) and B (n=3). They had both cervical and thoracic injuries. Written informed assent and consent was obtained under the protocol approved by IRB. Imaging: Subjects underwent scans using 3T Verio MR scanner. The protocol consisted of conventional T1- and T2-weighted scans and axial DTI scans based on the inner field of view sequence4 to cover the cervical (C1-upper thoracic region) and thoracic (upper thoracic region-L1) SC. The imaging parameters included: 20 diffusion directions, 6 b0 acquisitions, b=800s/mm2, voxel size=0.8x0.8x6mm3, axial slices=40, TR=7900ms, TE=110ms, and TA=8:49min. Data analysis: After motion correction5 and tensor estimation6, ROIs were manually drawn to extract information from the whole cord and SC white matter (WM) areas: ventral, dorsal, and both right and left lateral regions by a board certified pediatric neuroradiologist (Fig. 1). DTI parameters; FA, MD, AD and RD were quantified at each intervertebral disk level and at the mid-vertebral body level of the cervical and thoracic SC in TD and SCI subjects. The axial gradient-echo T2-weighted MR image was used as a reference for ROI placement on WM regions. For each SCI subject, the regions relative to injury were identified. First, the level of injury was identified using the conventional MR images. The cephalad region was defined as region above the level of injury and caudal region was defined as region below the level of injury. We chose to separate the cephalad and caudal regions into approximately three equal parts. These three equal parts were distal third, middle third and proximal third cephalad and caudal to the level of injury. DTI parameters for each WM region were measured at the levels distal third, middle third and proximal third cephalad and caudal relative to injury. Statistical analysis: A t-test was performed to assess the differences between TD and SCI in regions cephalad and caudal to injury. A p value <0.05 was considered statistically significant.Results
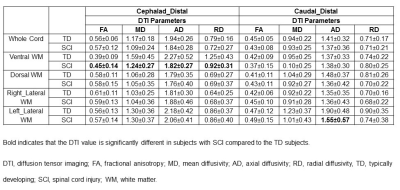
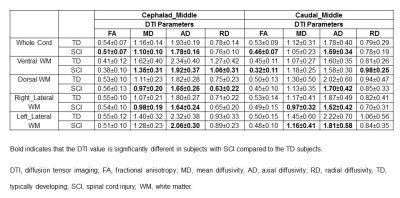
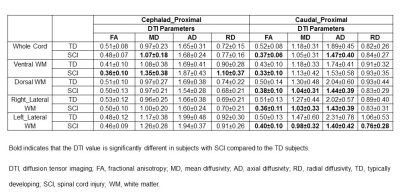
Results: FA, MD, AD and RD showed significant changes in ventral WM in SCI subjects compared to TD at distal levels cephalad to the injury (Table 1). MD and AD was significantly lower in whole cord, ventral, dorsal and right lateral WM in SCI subjects compared to TD at middle levels cephalad to the injury (Table 2). FA was significantly lower in all the WM regions and whole cord in SCI subjects at proximal levels caudal to the injury compared to TD (Table 3). MD and AD also showed significant changes in dorsal, right and left lateral WM at proximal levels caudal to the injury (Table 3).Discussion
Discussion: An apriori theory was that there is more abnormality in the adjacent perilesional (proximal) WM in comparison to the more distal WM above and below the lesion. In this study, we found that WM regions proximal to the injury site demonstrated altered diffusion values compared with the distal regions of the cord below (caudal) the level of injury. However, we also found significant DTI changes in WM regions at the middle levels above (cephalad) the injury. In addition, we also found ventral WM changes in the distal cephalad, however no changes were observed when whole cord ROI’s was used. These variations in diffusivity measures away from the injury site may reflect changes in tissue structure.Conclusion
Conclusion: We demonstrated changes in FA and AD in WM regions at levels both cephalad and caudal to the injury site. In contrast, conventional MRI showed no cord signal abnormalities. This suggests that FA and AD has the potential to be sensitive marker of true extent of cord injury and might be useful in detecting remote injuries.Acknowledgements
This work was supported by National Institute of Neurological Disorders of the National Institutes of Health under award number R01NS079635 (Mohamed FB and Mulcahey MJ, PI).References
References: 1. Ellingson BM, Ulmer JL, Kurpad SN et al. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR 2008;29:1976-1982. 2. Koskinen E, Brander A, Hakulinen U, et al. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma. 2013;30:1587-1595. 3. Petersen JA, Wilm BJ, von Meyenburg J et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012;29:1556-1566. 4. Finsterbusch J. Improving the performance of diffusion-weighted inner field-of-view echo-planar imaging based on 2D-selective radiofrequency excitations by tilting the excitation plane. JMRI 2012;35:984–992. 5. Middleton DM, Mohamed FB, Barakat N et al. An investigation of motion correction algorithms for pediatric spinal cord DTI in healthy subjects and patients with spinal cord injury. MRI 2014;32:433-39. 6. Saksena S, Middleton DM, Krisa L et al. Diffusion tensor imaging of the normal cervical and thoracic pediatric spinal cord. AJNR Jul 14. [Epub ahead of print]Figures