3076
Children with Sickle Cell Disease treated with Hydroxyurea show increased CVR and White Matter Integrity: a quantitative MRI study1SickKids Hospital, Toronto, ON, Canada, 2University of Toronto, Toronto, ON, Canada
Synopsis
Sickle cell disease (SCD) is a devastating genetic blood disorder leading to chronic anemia and cerebral infarctions. We sought to assess microstructural properties in the WM using diffusion tensor MRI and compare them to measures of cerebrovascular reactivity (CVR). Specifically, we investigated the effect of hydroxyurea (HU) treatment in SCD. Our results show that non-HU patients had increased skew and kurtosis of mean diffusivity in the WM compared to HU patients and healthy controls, and these parameters were correlated to WM CVR in this group. This suggests HU may have beneficial effects on WM microstructural integrity in patients with SCD.
Introduction
Sickle cell disease (SCD) is a devastating genetic blood disorder characterized by the deformation of erythrocytes, leading to vaso-occlusion, intravascular hemolysis, and hypoxemia. Children with SCD are at an increased risk of stroke, impaired cognitive ability, and early onset of dementia.1-4 Moreover, these impairments have been linked to disruption in the white matter (WM) integrity in children with SCD.5
Diffusion-tensor imaging (DTI) allows for microstructural analysis of WM by characterizing diffusion of water molecules. One of the primary measures of DTI is mean diffusivity (MD), indicating the total diffusion in a voxel. In SCD, widespread MD increases in WM have been observed and interpreted as demyelination and axonal injury.6
We have previously demonstrated that cerebrovascular reactivity (CVR), a measure of vascular reserve, is diminished in SCD patients.7 We have also demonstrated that therapy with hydroxyurea (HU) results in significant CVR improvement in children with SCD,8 but its impact on WM microstructural integrity is not yet clear. We therefore assessed the relationship between CVR and MD histogram parameters obtained from DTI in the WM of pediatric SCD patients with and without HU therapy.
Methods
Twenty-three SCD patients (13M/10F; average age 14.1 ± 2.6 years) with no history of overt stroke and not on chronic transfusion therapy were included in the study. Eleven patients were on HU therapy (HU group) and 12 patients were not (non-HU group). Patients were imaged on a clinical 3T MRI system (Siemens Medical Solutions, Germany) using a 32-channel head coil. Data from 10 healthy controls (6M/4F; average age 14.0 ± 2.4 years) were also collected. Imaging included structural T1, T2, and diffusion weighted sequences, as well as blood-oxygen-level dependent (BOLD) CVR scan. Structural data were reviewed by a radiologist to detect tissue infarction, while the CVR and DTI data were further processed on a separate workstation.
CVR was calculated from the BOLD acquisition (TR/TE=2000/30ms, FOV=220mm, matrix=64×64, slices=25, thickness=4.5mm), which ran in parallel with a CO2 block-design breathing challenge.7 In brief, reactivity was computed based on the voxel-wise BOLD signal change was correlated to the partial pressures of CO2 sampled at the end of each exhaled breath. A WM mask, created from the T1 image, was applied to calculate mean CVR across the entire WM.
DTI data were acquired with an echo-planar spin-echo sequence (TR/TE=9000/90ms, FOV=244mm, matrix=122×122, 30 directions, b-value=0,1000s/mm2). The data were processed through an automated skeletonization pipeline to produce masks along the primary WM fiber tracts in the brain.8 Evaluation of MD histogram parameters within the masks (mean, median, 5th percentile, 25th percentile, 75th percentile, and 95th percentile) was performed using FSL, and skew and kurtosis were analyzed using in-house MATLAB scripts.10
Statistical analyses were carried out in GraphPad Prism.11 One-way ANOVAs were used to examine significant differences (p<0.05) in the histogram parameters and CVR measurements in WM between groups. Linear regression analysis was performed to investigate relationships between MD histogram parameters and CVR data.
Results
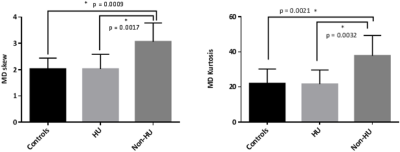
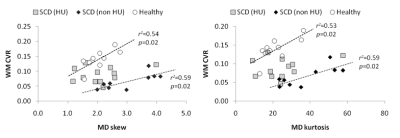
A significant decrease in WM CVR was observed in both HU and non-HU patients (0.067±0.026 and 0.089±0.026) when compared to healthy controls (1.397±0.031; p<0.001 for both). ANOVA revealed significant elevation in MD histogram skew and kurtosis of non-HU subjects when compared to either controls or HU patients (Figure 1). There were no significant differences among the remaining MD histogram parameters between controls, HU, and non-HU patient groups. WM lesions were identified in 10 patients (3 non-HU, 7 HU), but their presence did not have a significant influence on histogram findings. WM CVR was also significantly associated with MD histogram skew and kurtosis in controls (skew: r2 = 0.54; p = 0.02; kurtosis: r2 = 0.53; p = 0.02) and non-HU patients (skew: r2 = 0.59; p = 0.02; kurtosis: r2 = 0.59; p = 0.02), as shown in Figure 2. This relationship was not present in HU-treated patients.Discussion/Conclusion
Our results demonstrate that pediatric SCD patients without HU treatment exhibit increased WM MD histogram kurtosis and skew across WM tracts. The higher values may indicate greater axonal injury and demyelination across the brain in patients who are not treated with HU. In contrast, patients on HU had MD histogram parameters that closer resembled those of healthy volunteers (Figure 1). The scatter plots in Figure 2 show that the relationship between WM CVR and MD parameters in the HU treated group is shifted away from the non-HU patient data towards the healthy control data points. As our sample size is limited, additional data are needed to substantiate these findings, and potentially relate observed changes in WM integrity to cognitive function.Acknowledgements
No acknowledgement found.References
1. Miller ST, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139:385-90.
2. DeBaun MR, et al. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587-96.
3. van der Land V, et al. Risk factor analysis of cerebral white matter hyperintensities in children with sickle cell disease. Br J Haematol. 2016;172:274-84.
4. Al-Jafar HA, et al. Neurological Complications in Sickle Cell Disease (SCD). Int J Clin Exp Neurol. 2016;4:9-18.1
5. Scantlebury N, et al. White matter integrity and core cognitive function in children diagnosed with sickle cell diseae. J Pediatr Hematol Oncol. 2011; 33(3):163-71.
6. Sun B, Brown RC, Hayes L, et al. White Matter Damage in Asymptomatic Patients with Sickle Cell Anemia: Screening with Diffusion Tensor Imaging. AJNR. 2012; 33(11):2043-9.
7. Kosinski PD, Croal PL, Leung J, et al. The Severity of Anaemia Depletes Cerebrovascular Dilatory Reserve in Children with Sickle Cell Disease: a Quantitative Magnetic Resonance Imaging Study. BJH. 2016; 176(2):280-7.
8. Kosinski PD, Croal PL, Leung J, et al. Transfusion Therapy and Hydroxyurea Improves Cerebrovascular Reserve and Perfusion in Children with Sickle Cell Anemia: An MRI Study. Blood. 2015;126:3397. Abstract.
9. Baykara E, Gesierich B, Adam R, et al. A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms. Ann Neurol. 2016; 80(4):581–92.
10. MATLAB 8.0 and Statistics Toolbox 8.1, The MathWorks, Inc., Natick, Massachusetts, United States.
11. GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.