3074
HyperSIFT: Temoporal Denoising of Hyperpolarized Data Improves SNR while Perserving Dynamic Information1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Molecular Pharmacology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Weill Cornell Medical College, New York, NY, United States
Synopsis
We apply a temporal denoising algorithm to hyperpolarized MRI. The SIFT method filters out temporal frequencies with low amplitudes, reducing noise while preserving dynamic information. We demonstrate this in a bioreactor setting, introducing hyperpolarized [1-13C] pyruvate to cells and human subjects and observing the conversion of pyruvate to lactate.
Introduction
Hyperpolarized (HP) MRI uses substrates that have been polarized far above thermal equilibrium (~10,000x), resulting in negligible background signal. The spectra are sparse in the chemical shift domain, with a few peaks at known chemical shift offsets representing the substrate and metabolic products. Furthermore, the hyperpolarized signal is smoothly varyingWhereas the hyperpolarized signal is strong and smoothly varying, the noise signal is weak and rapidly varying. Therefore, it is possible to improve the SNR of temporal data using the SIFT technique(1).Methods
Data were Fourier transformed and a range of chemical shift frequencies selected to identify a noise region (0-150 ppm). The data were Fourier transformed in the temporal dimension and threshold was set at 2xSTD of the temporal spectra in the noise region; values below the threshold were set to zero. The value at the zero frequency was kept to preserve the baseline. Since real peaks have broad support, isolated values were removed using erosion and dilation functions. The resulting denoised data were inverse Fourier transformed to generate denoised dynamic spectra (Fig1).
Dynamic metabolic data were generated by introducing
HP [1-13C] pyruvate to breast cancer cells, as previously described
(2). [1-13C]pyruvic acid was polarized in a SPINlab hyperpolarizer
(GE). 13C NMR data were acquired on a 1T NMR spectrometer (Magritek) and human data were acquired on a 3T MRI (GE).
Results
Fig2 shows data with good SNR (the lactate signal is on the order of the pyruvate hydrate signal. The temporal spectrum is shown for the lactate peak (B), with a tall, broad peak at the low frequencies. There are a few noise peaks visible at the higher frequencies of the original data that are reduced at in the denoised data. The reduction in noise makes the lactate peaks more easily distinguishable from the surrounding noise. Fig3 shows data with much lower signal in the lactate peaks. In this case, there are relatively larger contributions from noise in the temporal spectrum that are removed by the SIFT algorithm. The dynamics of the lactate production are more clearly observed following denoising.
The dynamics from cells treated with a control and an AKT inhibitor are shown in Fig4. The difference in lactate production is more clearly distinguished in the denoised data. The SNR of lactate in both the control and treated group is between 0 and 5. In the denoised data the lactate SNR is 12-30 in the control group and 7-16 in the treatment group.
In a test/re-test experiment in a human prostate, the polarization varied between the two injections (35% and 22%) resulting in differerent SNR in the two datasets. Following denoising, the two data sets show comparable SNR, and lactate peaks that were at the noise level become more conspicuous. This demonstrates that this denoising method can be successfully used in vivo and with spatially resolved spectra.
Discussion
The heights of large peaks (e.g., pyruvate and the hydrate) do not change; the improvement in SNR is due to the reduction in the amplitude of the noise. For smaller peaks (e.g., lactate), the amplitude of an individual time point may change do the smoothing of the temporal dynamic, though the largest effect on improving peak detection Is the reduction of the noise at the base of the peak. There is no broadening of the peaks as there is with spectral apodization because the filtering is applied in the temporal, not spectral domain. The improvement in SNR allows us to detect peaks with lower SNR, such as the natural abundance [2-13C]pyruvate in the cell data and bicarbonate in the human prostate data.
There may be an artifact at the edges of the dynamics due to the periodic nature of the discrete Fourier transform. This is most significant when there is a sharp transition, such as for the large pyruvate signal in the first time point, which results in an artificially lower signal in the early time points. Beginning the acquisition prior to the introduction of the hyperpolarized substrate and acquiring until the signal is near zero so there is a smooth transition at the boundaries can minimize the decrease in signal. Alternatively, the early time points could be excluded from quantification.
Conclusion
The SIFT algorithm is a simple method for improving the SNR of hyperpolarized spectra. For sparse spectra, it is possible to perform the denoising semi-automatically, specifying only a chemical shift region with no expected peaks. Because this method utilizes dynamic information, it is possible to identify peaks with an SNR of ~1 in the original data. We have demonstrated this method in both in vitro and in vivo, laying the foundation for its rapid incorporation into future human HP MRI studies.Acknowledgements
NIH R00 EB014328, R01 CA195476 and S10 OD016422; NIH/NCI Cancer Center Support Grant P30 CA008748; Geoffrey Beene Cancer Research Center, Center for Molecular Imaging and Nanotechnology at MSKCC, The Center for Experimental Therapeutics at MSKCC, Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Sir Peter Michael Foundation.References
1 Doyle M, Chapman, BL, Balschi JA, Pohost GM. SIFT, a Postprocessing Method That Increases the Signal-to-Noise ratio of Spectra Which Vary in Time. J Mag Reson B. 1994;103:128-133.
2 Keshari KR, Kurhanewicz J, Jeffries RE, Wilson DM, Dewar BJ, Van Criekinge M, Zierhut M, Vigneron DM, Macdonald JM. Hyperpolarized 13C spectroscopy and an NMR-compatible bioreactor system for the investigation of real-time cellular metabolism. Mag Reson Med. 2010;63(2):322-329.
Chen AP, Cunningham CH, Ozturk‐Isik E, Xu D, Hurd RE, Kelley DA, Pauly JM, Kurhanewicz J, Nelson SJ, Vigneron DB. High‐speed 3T MR spectroscopic imaging of prostate with flyback echo‐planar encoding, J. Magn. Reson. Imaging 2007; 25:1288–1292.
Figures
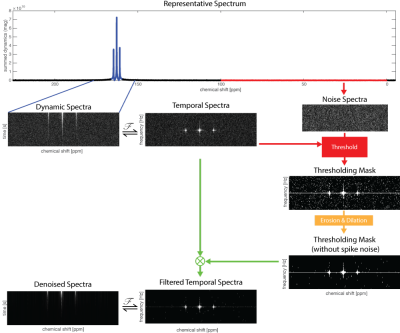
Figure 1 - SIFT algorithm
Spectroscopic data are Fourier transformed in the temporal domain. The standard deviation of the noise is used to threshold the temporal spectra to remove low-intensity values. HP 13C urea data acquired to measure the T1 decay (5° flip angle, 3s TR) are used to demonstrate the method.
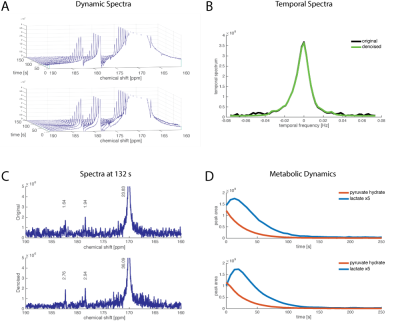
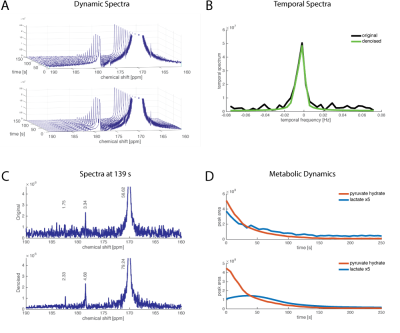
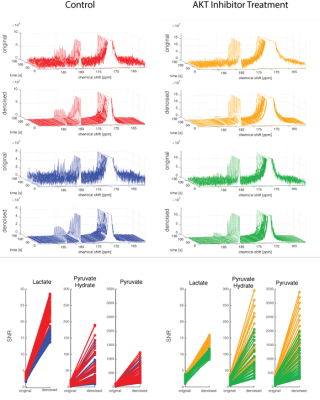
Figure 4: Cancer Cell Treatment Data
HP [1-13C]pyruvate was dissolved and mixed with 30x106 cells resulting in a final concentration of 10mM. 13C NMR data were acquired with TR=3 s, 10° flip angle. The treatment group was treated with 1uM MK2206, an AKT inhibitor. With the higher SNR in the denoised data, the difference in lactate production is more clearly observed.
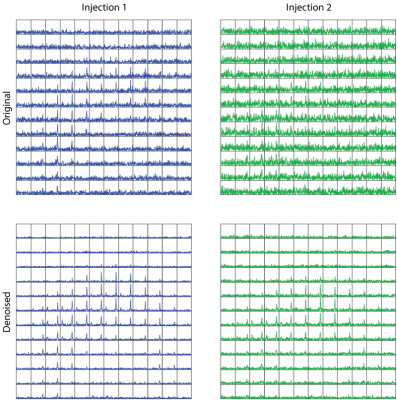
Figure 5 Human data
A patient was given test/re-test injections of 250mM pyruvate (0.43mL/kg) followed by a saline flush. 2D dynamic imaging began 5s after the saline flush. 2D spectra were acquired every 5s using an EPSI sequence with a variable flip angle scheme (5-20°) (3). Though the polarization level varies between the two injections (35% and 22%) the SNR is comparable following denoising.