3072
Hyperpolarized 13C chemical shift imaging of transient focal ischemia reperfusion injury in developing rat brain1Dept. of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Dept. of Pediatrics, University of California San Francisco, San Francisco, CA, United States, 3Joint UCSF/UC Berkeley Graduate Group in Bioengineering, San Francisco, CA, United States
Synopsis
We investigated the use of hyperpolarized [1-13C]pyruvate magnetic resonance chemical shift imaging in monitoring energy metabolism in developing rat brain following transient focal ischemia-reperfusion injury, which is the most common form of stroke in neonates. We show that the conversion from [1-13C]pyruvate to [1-13C]lactate was higher in the injured cerebral hemisphere as compared with that in the contralateral hemisphere, which lasted for up to 7 days after the ischemia-reperfusion injury. This phenomenon can be potentially used as a biomarker to facilitate long-term prognosis, characterize therapeutic responses and study the mechanisms of injury repair in neonates with transient focal ischemic stroke.
INTRODUCTION
Stroke during the neonatal period occurs in as many as 1 in 2,300 live births, and leads to lifelong effects such as increased incidence of cerebral palsy, epilepsy, mental retardation, etc. Transient ischemia-reperfusion is the most common form of stroke in neonates. This research project aims to use hyperpolarized [1-13C]pyruvate magnetic resonance chemical shift imaging (CSI) to study the longitudinal metabolic changes in developing rat brain after transient ischemia-reperfusion brain injury, which was induced using a highly-reproducible transient middle cerebral artery occlusion (tMCAO)-reperfusion model at postnatal day 10 (P10) (1).METHODS
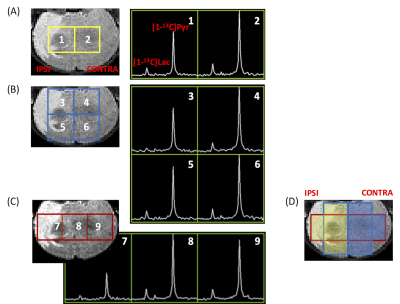
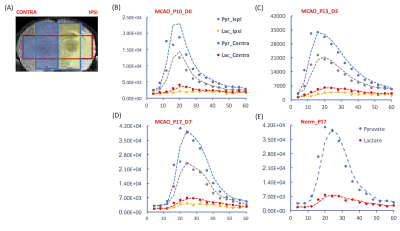
Animals: Three Sprague-Dawley rats (19-21 g) from the same litter were subjected to transient occlusion of middle carotid artery for 3 hrs at P10. One rat was imaged immediately after reperfusion (P10), and 7 days after reperfusion (i.e., postnatal day 17, P17). Another rat was imaged 3 days after reperfusion (i.e., postnatal day 13, P13). One normal control rat from the same litter was imaged at P17. Data acquisition: T2-weighted images were acquired for assessing the lesion and serving as the anatomical reference for hyperpolarized 13C CSI. For 13C CSI, 48 μL of [1-13C]pyruvate compound was hyperpolarized using a testbed polarizer (Oxford Instruments) for one hour (2). After dissolution, the hyperpolarized [1-13C]pyruvate was rapidly dissolved in 4.5 mL buffer, resulting in a 160 mM isotonic [1-13C]pyruvate solution (pH ~7.0). A bolus of 150 μL of the 160 mM [1-13C]pyruvate solution was injected over 12 seconds to each rat via a tail vein catheter that was prefilled with 150 μL heparinized saline. As a result, totally 300 μL liquid was injected to each rat over 12 seconds. From the beginning of tail vein injection, 2D dynamic CSI 13C data were acquired on a 3T Bruker small-bore MR scanner with a 40-mm 1H-13C quadrature coil using: repetition time/echo time = 66.4 ms/1.24 ms; SW 2000Hz; 128 points; 8 x 8 voxels, 4 sec resolution; FA = 10°; FOV= 30 x 30 mm²; 5 mm thickness. The first 15 spectra were included for analysis in this study. Data analysis: The 2D CSI data were reconstructed in three ways, resulting in two, three or four voxels covering largely pure brain tissue (Figs. 1A-1C). To plot dynamic curves, the [1-13C]pyruvate and [1-13C]lactate levels at each time point were calculated as the weighted sum of magnitude integrates from 3.5 voxels for either ipsilateral or contralateral hemisphere (Fig.1D). Averaged signals from both hemispheres were presented for the normal brain. The total [1-13C]pyruvate and [1-13C]lactate levels in either hemisphere were calculated as the sum of magnitude integrals over time.RESULTS AND DISCUSSION
By reconstructing the 2D CSI data in multiple ways, it is possible to sample signals from the majority of each hemisphere (shaded areas in Fig. 1D). All voxels in the ipsilateral hemisphere had lower [1-13C]pyruvate level and lower [1-13C]lactate level than those in the contralateral hemisphere (Fig. 1D, P17). Immediately after reperfusion, in the ipsilateral hemisphere, the [1-13C]lactate peak was only clearly shown in one spectrum acquired at 20 seconds after injection.
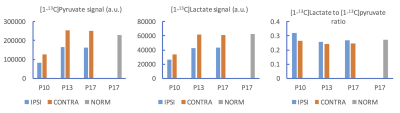
On the dynamic curves, the [1-13C]pyruvate level of the ipsilateral hemisphere was lower than that of the contralateral hemisphere at all time points after tMCAO (Figs. 2B-2D), consistent with the compromised perfusion to the ipsilateral hemisphere following focal stroke. Meanwhile, the decay of [1-13C]lactate level in the ipsilateral hemisphere, after it reached peak, appeared slower than that in the contralateral hemisphere (Figs.2C-2D). This might have led to the higher [1-13C]lactate to [1-13C]pyruvate ratio in the ipsilateral hemisphere than in the contralateral hemisphere (Figs. 3B-3C). Unlike in adult brain, lactate can serve as an important energy source in premature brain (3). Our observation could suggest increased pyruvate to lactate conversion or impaired lactate consumption as an energy source in the injured neonatal brain. Due to the extremely low [1-13C]lactate level in the ipsilateral hemisphere in the rat imaged immediately after tMCAO, the [1-13C]lactate to [1-13C]pyruvate ratio might not provide much information, and need to be further investigated. The reproducibility of the presented results will be further tested with a larger sample size.
CONCLUSION
By repeated reconstruction of the intrinsically low-spatial-resolution 2D dynamic 13C CSI data in multiple ways, we demonstrated the quantification of signals from the majority of the neonatal rat brain imaged using an 8 x 8 matrix size and a field of view of 30 x 30 mm2. The characteristics of the [1-13C]lactate signal dynamic curve following [1-13C]pyruvate injection and the [1-13C]lactate to [1-13C]pyruvate ratio may both provide insights for energy metabolism following neonatal transient ischemic stroke.Acknowledgements
The authors acknowledge grant support from NIH ( NIH R35NS097299, K08 NS064094).References
1. Larpthaveesarp A, Gonzalez FF. Transient Middle Cerebral Artery Occlusion Model of Neonatal Stroke in P10 Rats. J Vis Exp 2017(122), e54830, doi:10.3791/54830 (2017).
2. Chen Y, Kim H, Bok R, Sukumar S, Mu X, Sheldon RA, Barkovich AJ, Ferriero DM, Xu D. Pyruvate to Lactate Metabolic Changes during Neurodevelopment Measured Dynamically Using Hyperpolarized 13C Imaging in Juvenile Murine Brain. Dev Neurosci 2016;38(1):34-40.
3. Kasischke K. Lactate fuels the neonatal brain. Front Neuroenergetics 2011;3:4.
Figures