3071
Comparison of Asymmetric and Symmetric K-space Sampling in EPI for 3D Time-Resolved Hyperpolarized 13C MRI with [1-13C]Pyruvate1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 3GE Healthcare, Toronto, ON, Canada
Synopsis
The minimum echo-time for hyperpolarized 13C echo-planar imaging can be reduced with partial sampling along the blipped direction in k-space. To investigate the extent to which echo-time shortening can improve signal-to-noise ratio, we’ve employed an experimental design that toggles between two different spatial encoding strategies during a time-resolved hyperpolarized [1-13C]pyruvate acquisition. Using clinically approved hardware with a pre-clinical animal model, we compared symmetric with asymmetric echo-planar imaging. Considerable signal-to-noise ratio gains for asymmetric vs symmetric sampling were observed without artifacts. On the basis of this study, our group will employ asymmetric sampling in our forthcoming human trials.
Introduction
Hyperpolarized (HP) 13C magnetic resonance imaging and spectroscopy with dynamic nuclear polarization (DNP) substrates is an emerging technology for monitoring cellular metabolism in vivo. Due to favourable DNP properties and metabolic significance in diseases such as heart failure and cancer, studies involving the substrate [1-13C]pyruvate are driving current progress in translating HP 13C MRI for clinical use, and several human trials have been reported.1-10
Some of our group’s forthcoming HP [1-13C]pyruvate trials focus on imaging solid tumours in a variety of patient cohorts, and utilize spectrally and spatially selective radio frequency (ssRF) excitation followed by 3D echo-planar imaging (EPI) readouts (2D EPI + phase encoding along the “slice” direction) to generate time-resolved, volumetric images of [1-13C]pyruvate and [1-13C]lactate.
In the ssRF + “fast-readout” regime, pulse durations can be on the order of tens of milliseconds, and this invariably extends the minimum echo-time (TE) in comparison to approaches that utilize non-selective RF excitation. Longer TE entails heavier T2* weighting and this can lead to degradation of signal-to-noise ratio (SNR). For a fixed RF pulse length, the minimum TE for ssRF + 3D EPI can be reduced considerably with partial sampling along the blipped direction in k-space.
To explore SNR performance of asymmetric vs symmetric k-space sampling in EPI, we’ve employed a previously described11 experimental design that toggles between two different spatial encoding strategies during a dynamic HP [1-13C]pyruvate acquisition. By interleaving the two readouts within a single injection rather than between successive experiments, potentially confounding variables such polarization, perfusion, injection timing and metabolism can be arguably eliminated. Here we present pre-clinical results comparing asymmetric vs symmetric EPI obtained with a HP 13C human breast imaging platform12.
Methods
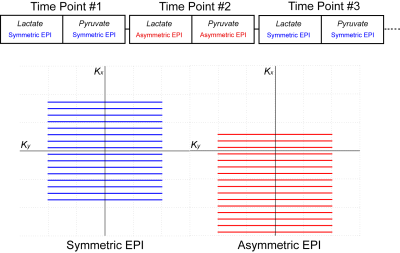
Symmetric EPI gradient waveforms were designed for 96×12 cm2 field-of-view at 7.5 mm in-plane resolution. For asymmetric EPI, the dephasing and rephasing trapezoids for the blipped gradient were redesigned to shift the sampling window in k-space by the equivalent of either 5 or 6 lines, reducing minimum TE by 7.6 and 9.2 ms, respectively.
Phase encoding was used for 24 cm coverage with 10 cm slice thickness. A tailored 18.8 ms ssRF pulse13 was designed for excitation. Waveforms were incorporated into a custom 3D gradient-echo sequence and were toggled according to figure 1. Odd and even time points were encoded with symmetric and asymmetric EPI, respectively.
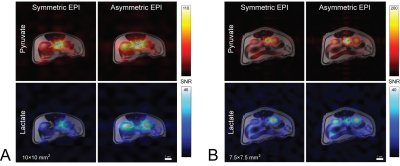
Imaging was performed on a GE MR750 3T MR scanner (GE Healthcare, Waukesha, WI) using the previously described 2-channel HP 13C breast imaging platform (figure 2). Two Sprague-Dawley rats were handled in accordance with our institutional animal care and use committee.
For rat A (480 g), the asymmetric EPI readout was shifted by 6 lines (~9.2 ms) and images were acquired at 10 mm isotropic resolution. For rat B (500 g), the readout was shifted by 5 lines (~7.6 ms) and the resolution was increased to 7.5×7.5×10 mm3.
Results & Discussion
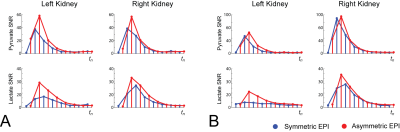
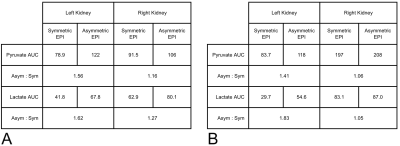
Time-integrated [1-13C]pyruvate and [1-13C]lactate overlays are displayed in figure 3. In both experiments, SNR within the right kidney was apparently similar for either EPI encoding method; however, there is a striking difference within the left kidney, with elevated SNR in volumes encoded with asymmetric EPI. A region-of-interest (ROI) analysis for the kidney signal was performed and is illustrated in figure 4. Area-under-the-curve (AUC) summary metrics of the ROI analysis are tabulated in figure 5.
In experiment A, the left/right kidney averaged SNR increase for [1-13C]pyruvate and [1-13C]lactate was 36% and 45%, respectively, and for experiment B, the increase was 24% and 44%. If the difference in SNR was primarily due to T2* dephasing during the readout, then the benefit in asymmetric EPI vs symmetric EPI would be expected to be higher in experiment A than experiment B, and this was observed. The voxel size used in experiment A was approximately 78% larger than in experiment B, which, all else equal, would contribute to increased intra-voxel dephasing. Additionally, 6 rather than 5 lines were skipped, providing experiment A with a shorter TE than experiment B. Despite the significantly larger voxel size in A vs. B, the total SNR was comparable, which underscores the advantage of comparing sequences within a single injection.
Conclusion
Asymmetric EPI is a viable method for shortening minimum TE in order to mitigate T2* related SNR reductions in the ssRF regime. In the cases studied, the SNR advantage of asymmetric-vs-symmetric EPI was in the range of 20-50%. A potential drawback of asymmetric-vs-symmetric EPI sampling is ringing artifacts, which were not observed here. On the basis of this study, our group will be employing asymmetric EPI in our forthcoming human trials.
Acknowledgements
The authors are thankful for funding from the Canadian Breast Cancer Foundation Ontario and the Canadian Cancer Society Research Institute and for assistance from Jennifer Barry and Yiping Gu for animal handling.
References
- Cunningham, Charles H., Justin YC Lau, Albert P. Chen, Benjamin J. Geraghty, William J. Perks, Idan Roifman, Graham A. Wright, and Kim A. Connelly. Hyperpolarized 13C Metabolic MRI of the Human Heart: Novelty and Significance." Circulation research 119, no. 11 (2016): 1177-1182.
- Chen, Albert P., Justin YC Lau, Benjamin J. Geraghty, William J. Perks, Idan Roifman, Graham A. Wright, Kim A. Connelly, and Charles H. Cunningham. “Hyperpolarized 13C MRS of the Human Heart.” In Proc 25th Annual Meeting ISMRM, Honolulu, HI, USA. 2017.
- Nelson, Sarah J., John Kurhanewicz, Daniel B. Vigneron, Peder EZ Larson, Andrea L. Harzstark, Marcus Ferrone, Mark van Criekinge et al. "Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate." Science translational medicine 5, no. 198 (2013): 198ra108-198ra108.
- Park, Ilwoo., Peder EZ Larson, Jeremy W. Gordon, Lucas Carvajal, Hsin-Yu Chen, Mark van Criekinge, Robert Bok, Jason C. Crane, Adam Elkhaled, Joanna Phillips, James B. Slater, Marcus Ferrone, John Kurhanewicz, Daniel B. Vigneron, Susan Chang, and Sarah J. Nelson. "Dynamic Hyperpolarized 13C Metabolic Imaging of Patients with Brain Tumors." In Proc 25th Annual Meeting ISMRM, Honolulu, HI, USA. 2017.
- Zhu, Zihan., Irene Marco-Rius, Michael A. Ohliger, Lucas Carvajal, Jeremy W. Gordon, Hsin-Yu Chen, Ilwoo Park, Peng Cao, Peter J. Shin, Eugene Milshteyn, Cornelius von Morze, Marcus Ferrone, James B. Slater, Zhen Wang, Peder EZ Larson, Rahul Aggarwal, Robert Bok, John Kurhanewicz, Pamela Munster, and Daniel B. Vigneron. "Hyperpolarized 13C Dynamic Breath-held Molecular Imaging to Detect Targeted Therapy Response in Patients with Liver Metastases." In Proc 25th Annual Meeting ISMRM, Honolulu, HI, USA. 2017.
- Chen, Hsin-Yu., Peder EZ Larson, Jeremy W. Gordon, Robert A. Bok, Marcus Ferrone, Mark van Criekinge, Lucas Carvajal, Peng Cao, Ilwoo Park, Rahul Aggarwal, Sarah J. Nelson, John Kurhanewicz, and Daniel B. Vigneron. “3D Dynamic Hyperpolarized 13C-Pyruvate MR Metabolic Imaging of Human Prostate Cancer” In Proc 24th Annual Meeting ISMRM, Singapore. 2016.
- Aggarwal, Rahul., Daniel B. Vigneron, and John Kurhanewicz. "Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer." European Urology (2017).
- Granlund, Kristin L., Elizabeth A. Morris, Hebert A. Vargas, Serge K. Lyashchenko, Phillip J. DeNoble, Virgilio A. Sacchini, Ramon A. Sosa, Matthew A. Kennedy, Duane Nicholson, YanWei W. Guo, Albert P. Chen, James Tropp, Hedvig Hricak and Kayvan A. Keshari. "First-in-woman study of in vivo breast cancer metabolism using hyperpolarized [1-13C]pyruvate." In Proc 24th Annual Meeting ISMRM, Singapore. 2016.
- Granlund, Kristin L., Hebert A. Vargas, Serge K. Lyashchenko, Phillip J. DeNoble, Vincent A. Laudone, James Eastham, Ramon A. Sosa, Matthew A. Kennedy, Duane Nicholson, YanWei W. Guo, Albert P. Chen, James Tropp, Hedvig Hricak, and Kayvan R. Keshari. “Metabolic Dynamics of Hyperpolarized [1-13C] Pyruvate in Human Prostate Cancer.” In Proc 24th Annual Meeting ISMRM, Singapore. 2016.
- Tyler, Damian., Oliver Rider, Michael Dodd, Angus AZ Lau, Andrew Lewis, Jack Miller, Mark Peterzan, Claire Trumper, and Stefan Neubauer. “Demonstrating the Randle Cycle In Vivo: Assessment of Physiological Alterations in Human Cardiac Metabolism Using Hyperpolarised 13C MR Spectroscopy” In Proc 25th Annual Meeting ISMRM, Honolulu, HI, USA. 2017.
- Geraghty, Benjamin J., Justin YC Lau, Albert P. Chen, William Dominguez-Viqueira, and Charles H. Cunningham. “SNR Comparison of EPI and Spiral 3D Time Resolved Imaging of Hyperpolarized [1-13C]Pyruvate and [1-13C]Lactate.” In Proc 23rd Annual Meeting ISMRM, Toronto, Ontario, Canada. 2015.
- Geraghty, Benjamin J., Justin YC Lau, Logi Vidarsson, William Dominguez-Viqueira, Albert P. Chen, and Charles H. Cunningham. “Platform for Hyperpolarized 13C MRI of Breast Cancer” In Proc 25th Annual Meeting ISMRM, Honolulu, HI, USA. 2017.
- Cunningham, Charles H., Albert P. Chen, Michael Lustig, Brian A. Hargreaves, Janine Lupo, Duan Xu, John Kurhanewicz et al. "Pulse sequence for dynamic volumetric imaging of hyperpolarized metabolic products." Journal of magnetic resonance 193, no. 1 (2008): 139-146.
Figures