3068
Non-invasive redox molecular imaging of atopic dermatitis using in vivo dynamic nuclear polarization MRIFuminori Hyodo1,2, Hinako Eto2, Gaku Tsuji2, Masutaka Furue2, and Masayuki Matsuo1
1Gifu University, Gifu, Japan, 2Kyushu University, Fukuoka, Japan
Synopsis
Atopic dermatitis (AD) is a chronic inflammatory condition with complex etiology. Redox imbalance caused by excessive oxidative stress has been shown to mediate disease activity of AD. We have established such a technique that can detect and visualize the redox status of the skin using in vivo dynamic nuclear polarization(DNP) MRI. We utilized an AD mouse model that was generated by repeated topical application of mite antigen in NC/Nga mice. We revealed that AD skin lesions demonstrated more rapid reduction rates of image intensity than normal skin, indicating that our technique can monitor oxidative stress in AD skin.
Introduction
Atopic dermatitis (AD) is an inflammatory skin disease that results from the interaction of genetic, environmental, and immunologic factors. Recent studies have reported that oxidative stress may play an important role in many skin diseases including skin aging and AD. In vivo dynamic nuclear polarization magnetic resonance imaging (DNP-MRI) is a method of free radical imaging in the living body. In this study, we have for the first time established the in vivo imaging of redox status of lesional skin in NC/Nga mice, the animal model of AD. This model is generated by repeated topical application of mite antigen, and shows similar disease characteristics to human AD. We have succeeded in the visualization of redox alterations in the AD skin lesions of these mice. Non-invasive monitoring of dermal tissue redox status by in vivo DNP-MRI could be of great value in understanding the progression of AD.Methods
Atopic dermatitis mouse model was made by topical application of Biostir AD ointment. These processes were repeated twice a week and clinical scores were evaluated by visual inspection. After four rounds of stimulation (2 weeks), the acute stage mouse model with AD-like lesions was established and designated acute-AD mice. The late stage mouse model, designated chronic-AD mice, required a total of eight rounds of stimulation (4 weeks). In vivo redox imaging was performed with an in vivo DNP-MRI system, constructed in our lab. As well as the in vivo DNP-MR imaging, mice were anesthetized with 2% isoflurane and placed on the stage in the supine position. Magnetic resonance images (sagittal plane) of chronic-AD mice (n = 3) and control mice (n = 3) by 1.5T animal MRI were obtained before and after (2 min and 20 min) subcutaneous injection of tempol isotonic solution (2.5 mM, 100 µl) to the head. The scanning conditions for the MRI experiment were as follows: flip angle, 90°; repetition time (TR) × echo time (TE), 500 × 10 ms; sagittal section; number of accumulation, 2; slice thickness, 1 mm; phase-encoding steps, 130; sampling number,196; FOV, 60 × 60 mm; and matrix size, 256 ×256 after reconstruction.Results
In vivo redox imaging of the skin was performed with an in vivo DNP-MRI system, constructed in our lab. A rectangular one-turn curved surface coil which was made for skin imaging in this study, was used for EPR irradiation. Tempol was used as a nitroxyl radical source for visualizing redox status of skin in this study and has high membrane permeability that also allows intracellular redox metabolism (Fig1a). We confirmed that the enhancement of MR image intensity was clearly confirmed at the region of administration in the dermal tissue of the mice, although there was no enhancement without EPR irradiation (DNP OFF) or after reduction of tempol radical to hydroxylamine form(Fig1b) . We confirmed that the distribution of tempol was similarly localized in both acute and chronic AD mice and control animals. Tempol induced enhancement of image intensity gradually decreased over time. The decay rates of image intensity in individual AD mice were significantly faster than those of control mice(Fig1c). , although there were no significant differences between acute- and chronic-AD mice. The redox maps of control mice showed uniformly low values; in contrast, those of AD skin lesions showed high values with inhomogeneity. These results suggest that reduction of tempol in AD mice is enhanced and thus may be caused by excessive oxidative stress.Discussion
Oxidative stress reportedly mediates and enhances inflammatory infiltrate and releases histamine, resulting in worsening skin symptoms. Because inflammation generally leads to faster reduction of nitroxyl radicals due to ROS overproduction in inflammatory cells, it is likely that faster reduction of tempol in AD skin lesions is related to excessive oxidants in the skin. In this study, redox alteration was observed not only in chronic-AD mice but also in acute-AD mice, and importantly, such early in vivo changes can be detected by DNP-MRI. As shown above, more severe acanthosis of the epidermis was observed in chronic-AD mice compared with acute-AD mice.Acknowledgements
We thank Dr. T. Naganuma and M. Nakao at Japan Redox Inc., and Prof. K. Ichikawa, Prof. H. Utsumi at the Innovation Center for Medical Redox Navigation at Kyushu University, for providing the custom in vivo DNP-MRI system.References
Eto H, Tsuji G, Chiba T, Furue M, Hyodo F. Non-invasive evaluation of atopic dermatitis based on redox status using in vivo dynamic nuclear polarization magnetic resonance imaging. Free Radic Biol Med. 2017 Feb;103:209-215.Figures
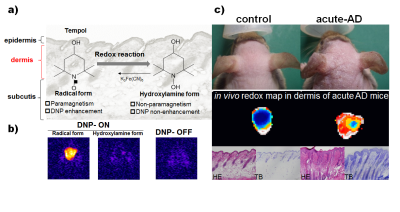
Fig.1 In vivo DNP-MRI imaging of
acute redox alteration on atopic dermatitis mouse model.
a)Schematics of tempol
redox reaction. b) The DNP-MRI images with(DNP ON) or without(DNP OFF)
EPR irradiation after s.c. injection of tempol
to normal mouse. c)Redox imaging of acute AD mice using in vivo
DNP-MRI and histological observation.