3066
Assessing γ-glutamyl transpeptidase activity in kidney using hyperpolarized γ-Glu-[1-13C]Gly1Laboratory for Functional and Metabolic Imaging, EPFL, Lausanne, Switzerland, 2Department of Chemistry and Biotechnology, The University of Tokyo, Tokyo, Japan, 3Department of Radiology, University of Geneva, Geneva, Switzerland, 4Department of Radiology, University of Lausanne, Lausanne, Switzerland, 5Centre for Biomedical Imaging, EPFL, Lausanne, Switzerland
Synopsis
Hyperpolarized γ-Glu-[1-13C]Gly provides a non-invasive means to detect γ-glutamyl transpeptidase (GGT) enzyme activity in vivo and indicates its potential for application in functional imaging. Since GGT is most abundant in the proximal tubules of the kidney, and since the properties of γ-Glu-[1-13C]Gly are suitable for in vivo hyperpolarized 13C metabolic analysis, it was proposed as a molecular probe to study kidney function. The aim of the present study is to identify the dose of γ-Glu-[1-13C]Gly that gives high NMR sensitivity in the unsaturated state of the GGT enzyme.
Introduction
The γ-glutamyl transpeptidase (GGT) enzyme plays a key role in the γ-glutamyl cycle by regulating the cellular levels of the antioxidant molecule glutathione [1]. It is involved in many physiological disorders related to oxidative stress, such as Parkinson’s disease and diabetes, and it is upregulated during inflammation and in several malignant tumors [2]. In a recent study [3], γ-Glu-[1-13C]Gly has been reported as a hyperpolarized (HP) probe to target GGT activity in vivo. It features sufficiently long 13C T1 values of both γ-Glu-[1-13C]Gly and its metabolic product [1-13C]Gly (30 s, 45 s in H2O at 9.4 T, RT) and a 13C chemical shift difference of 4.3 ppm at physiological pH [3]. Since GGT is widely expressed in the proximal tubules of the kidney [1], the aim of the present study is to show if this molecular probe can be used to study kidney function. As a first step, different doses of hyperpolarized substrate were administered to investigate the saturation of the GGT enzyme in the kidney.Methods
γ-Glu-[1-13C]Gly was polarized with the stable trityl radical OX63. The polarization transfer from the electron spin on the radical to the 13C nuclear spin on γ-Glu-[1-13C]Gly was achieved in a custom-designed DNP polarizer (7T) using microwave irradiation at 196.59 GHz and 50 mW. After polarization at 1.1 K, γ-Glu-[1-13C]Gly was dissolved with 5.5 ml preheated deuterated phosphate and sodium chloride buffer (~ pH 7). Sprague-Dawley rats (male, 250-300g) were anesthetized with isoflurane. A femoral vein and artery were catheterized for substrate administration and IBP measurements, and their physiology was monitored during the entire experiment. The acquisition was carried out on a 9.4 T / 31 cm animal scanner (Varian/Magnex) using a home-built surface coil consisting of a 1H coil and a 13C coil driven in quadrature. The 13C MRS measurements of the right kidney had a repetition time of 3 s and 30° BIR4 adiabatic RF excitation pulses with proton decoupling were applied. As a first approach to analyze the HP data, the model used in [4] was applied. Herein, the initial reaction rates v0,GGT were calculated by multiplying the kinetic rate constants with the corresponding substrate concentrations, in which the kinetic rate constant is the product of the 13C longitudinal relaxation rate of glycine (~45s) and the ratio of the integrated γ-Glu-[1-13C]Gly and [1-13C]Gly signal amplitude.Results and Discussion
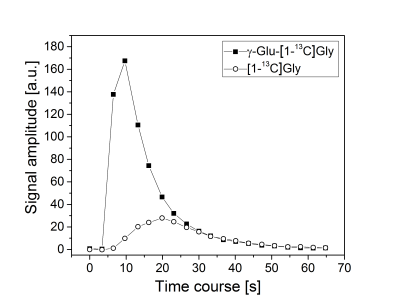
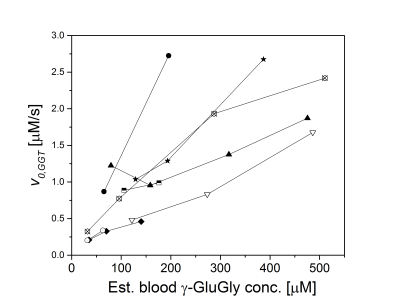
Benefiting from a narrow spectral linewidth of the hyperpolarized signal (~20 Hz, FWHM), conversion of γ-Glu-[1-13C]Gly to [1-13C]Gly was measurable down to an estimated blood concentration of 32 μM. Fig. 1 shows the temporal development of the conversion of hyperpolarized γ-Glu-[1-13C]Gly (~190 µM) to its metabolic product [1-13C]Gly. To address the possibility of substrate saturation of the GGT enzyme in the kidney, different doses of γ-Glu-[1-13C]Gly were administered, corresponding to a blood concentration range of 31 to 500 µM. Fig. 2 shows the dependence of the initial reaction rates v0,GGT on the hyperpolarized γ-Glu-[1-13C]Gly concentration in 8 rats. The variability of the initial reactions rates between animals is high for all doses of administered γ-Glu-[1-13C]Gly. The rate, however, was proportional with the dose in 7 rats, saturation of the GGT enzyme cannot be seen in the dosage range tested.Conclusion
This study shows that HP γ-GluGly for senses GGT activity with excellent NMR sensitivity and that a broad range of substrate concentrations can be applied to study kidney function. To understand better the distribution of the initial reaction rates and to estimate the dose required to saturate the GGT enzyme, a broader range of substrate doses will be tested, along with simultaneous functional quantification.Acknowledgements
This work was supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) within the Marie Curie Initial Training Network EUROPOL project (n° SERI: 15.0164), and by the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenards and Jeantet Foundations.References
[1] M.H. Hanigan et al, Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma, J.Histochem. Cytochem. 1996, 44, 1101 – 1108.
[2] I Castellano and A. Merlino, Gamma-Glutamyl Transpeptidases, SpringerBriefs in Biochemistry and Molecular Biology, 2013
[3] T. Nishihara and H. A. I. Yoshihara et al., Direct Monitoring of Gamma-Glutamyl Transpeptidase Activity In Vivo Using a Hyperpolarized 13C-Labeled Molecular Probe, Angew. Chem. Int. Ed. 2016, 55, 10626-10629
[4] J. A.M. Bastiaansen et al., In vivo enzymatic activity of acetylCoA synthetase in skeletal muscle revealed by 13C turnover from hyperpolarized [1-13C]acetate to [1-13C]acetylcarnitine, Biochimica et Biophysica Acta, 2013, 1830, 4171-4178
Figures