3061
In vitro and in vivo 13C metabolic imaging of pyruvate to lactate conversion with high spatial and temporal resolution using a me-bSSFP sequence1Department of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research (DKTK), Partnersite Freiburg, German Center for Cancer Research (DKFZ), Heidelberg, Germany, 3Department of Nuclear Medicine, Klinikum rechts der Isar, Technical University of Munich, München, Germany, 4Department of Clinical Physiology, Nuclear Medicine & PET, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark, 5Medical Radiation Physics, Malmö University Hospital, Lund University, Malmö, Sweden, 6Department of Electrical Engineering, Technical University of Denmark, Copenhagen, Denmark, 7Section for Biomedical Imaging, Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein, University of Kiel, Kiel, Germany
Synopsis
In order to use the transient signal of hyperpolarized tracers and their metabolites efficiently, dedicated imaging sequences are required. Here, we present a multi-echo bSSFP sequence with Dixon-based iterative reconstruction to obtain metabolite maps of hyperpolarized [1‑13C]pyruvate and the product of an enzymatic conversion [1-13C]lactate on a human 3T PET-MRI system in vitro and in vivo. When comparing to other methods (i.e. CSI and non-localized NMR spectra) we found that me-bSSFP provides good metabolite separation and reliable quantitative kinetic data more than 16 times faster than CSI (350 ms vs. 5.8 s), while consuming a similar amount of hyperpolarized magnetization.
Introduction
MRI with hyperpolarized tracers has enabled new diagnostic applications, e.g. for cancer research. However, the signal is transient and dedicated sequences are required for efficient use. Here, we present an optimized multi-echo balanced steady state free precession (me-bSSFP)1,2 sequence to image augmented lactate dehydrogenase (LDH) activity of subcutaneous breast cancer tumors in rats at a clinical PET/MR scanner. We validated the sequence with hyperpolarized 1D 13C-NMR measurements and compared it to a chemical shift imaging (CSI) sequence. Me-bSSFP enables in vitro and in vivo short image acquisition times at high spatial resolution and multiple chemical shifts simultaneously, and thus makes fast dynamic imaging possible to quantify metabolite conversion kinetics.Methods
[1-13C]pyruvate (HP-py) hyperpolarized with by DNP (Hypersense, Oxford Instruments) was dissolved in 100 mM PBS buffer and added either to 5 mL glass vials or 5 mm NMR tubes filled with aqueous and buffered solutions containing constant amounts of nicotinamide adenine dinucleotide (NADH) and increasing amounts of LDH according to the scheme in Table 1. For in vivo experiments, HP-py was injected approx. 24 s after dissolution via tail-vein into MAT-B-III bearing rats (female Fischer344, 1 mL, 90 mM HP-py, pH7.3)).
NMR 13C-spectroscopy was performed at a benchtop NMR spectrometer (Spinsolve, Magritek) with a pulse-acquire sequence. 13C-MRI with me-bSSFP and CSI (Table 2) was conducted on a clinical 3T PET-MRI system (Biograph, Siemens) equipped with a 1H-13C surface coil (Rapid Biomedical). A thermally polarized model solution (MS) containing [1-13C]lactate (TP-lac): 2.23 M, 0.15%(w/v) NaN3, was used for quality control and adjustments.
Zero-filling (2x) and Gaussian windowing was applied to CSI and me-bSSFP. Me-bSSFP images were reconstructed with a Dixon based iterative reconstruction4–6, and CSI data was Fourier transformed in time domain and peaks were integrated, to obtain frequency-selective metabolite maps at -186 Hz (HP-py) and +186 Hz (HP-lac). Signal-to-noise (SNR) ratios were calculated by dividing the mean signal by the standard deviation of selected regions of interest (ROI). NMR spectra were apodized by 5 Hz and quantified by integration of HP-py and HP-lac peaks. The py→lac conversion was quantified as area under the curve (AUC) ratio7 by dividing the AUC of the lactate signal by that of pyruvate.
Results
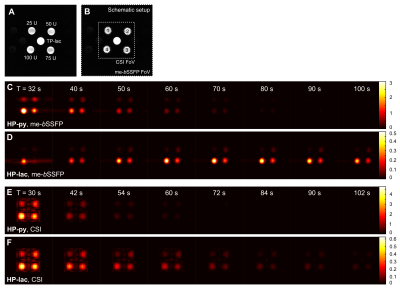
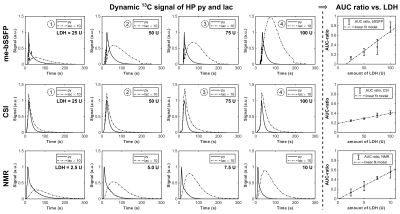
In vitro assays with increasing LDH concentrations were performed with time-resolved 1D NMR (control) and time- and spatially-resolved me-bSSFP and CSI. We were able to obtain a two-fold better spatial resolution and a six-fold higher temporal resolution with me-bSSFP than with CSI (0.35s vs. 5.8s per image). Although me-bSSFP used a two-fold-higher flip angle, SNR after 50 s acquisition and later was better than with CSI (Figure 1).
All methods exhibited a linear response of the AUC ratio to LDH concentration (Figure 2). However, AUC ratios acquired with me-bSSFP did not show a significant difference to control measurements with NMR, while this was not the case for CSI. This observation is also apparent by eye in the dynamic data (Figure 2).
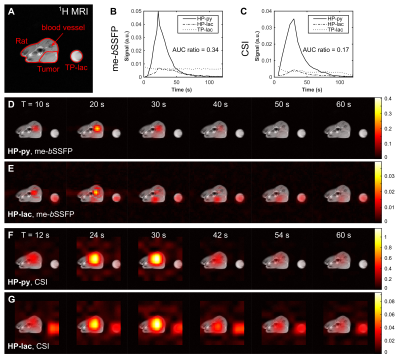
In vivo time-resolved axial metabolic maps of tumor-bearing rats were successfully acquired with CSI and me-bSSFP after the injection of HP-py at a clinical PET/MR scanner (Figure 3). As observed for in vitro experiments, me-bSSFP provided higher temporal and spatial resolution. HP-py and HP-lac signals are inhomogeneously distributed in the tumor, suggesting tumor heterogeneity. Spatial separation of tumor and healthy tissue was difficult for CSI because of the limited spatial resolution. The difference of the AUC ratio measured in vivo with me-bSSFP and CSI (0.34 and 0.17) replicates the discrepancy of the quantitative results from in vitro.
Discussion
In these experiments, me-bSSFP out performs CSI in terms of spatial and temporal resolution. Both exhibit some artifacts when HP-py signal is high. The efficient magnetization usage of the me-bSSFP enables the acquisition of more data in the same time window with good metabolite separation, ultimately resulting in more reliable (physiological) metabolite conversion curves, shown by the comparison to NMR. The AUC analysis underlines this statement. We showed in vitro, that LDH activity can be accurately quantified with me-bSSFP, but not with the used CSI protocol. In vivo, tumor pyruvate and lactate signal could be better distinguished from healthy tissue with me-bSSFP.Conclusion & Outlook
We developed and validated a fast me-bSSFP protocol for the application to quantify LDH activity of a pre-clinical breast cancer model in vivo at a clinical PET/MR system. Using this method, various tumor models - even of sizes closer to humans - can be characterized, potentially accelerating the translation of 13C hyperpolarized metabolic mapping to the clinic. Fast 3D 13C metabolic imaging with this method is also possible with only minor adjustments to the protocol.Acknowledgements
JBH wishes to acknowledge support by the DFG Emmy Noether program (HO4604/2-1)References
1. Leupold, J. et al. Fast chemical shift mapping with multiecho balanced SSFP. Magn. Reson. Mater. Phys. Biol. Med. 19, 267–273 (2006).
2. Leupold, J. et al. Fast multiecho balanced SSFP metabolite mapping of 1H and hyperpolarized 13C compounds. Magn. Reson. Mater. Phys. Biol. Med. 22, 251–256 (2009).
3. Abragam, A. & Goldman, M. Principles of dynamic nuclear polarisation. Rep. Prog. Phys. 41, 395 (1978).
4. Reeder, S. B. et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn. Reson. Med. 51, 35–45 (2004).
5. Reeder, S. B. et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): Application with fast spin-echo imaging. Magn. Reson. Med. 54, 636–644 (2005).
6. Yu, H. et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J. Magn. Reson. Imaging 26, 1153–1161 (2007).
7. Daniels, C. J. et al. A comparison of quantitative methods for clinical imaging with hyperpolarized 13C-pyruvate. NMR Biomed. 29, 387–399 (2016).
Figures
Table 2: Sequence parameters for 13C NMR at 1 T and for 13C MRI at 3 T.