3059
In vivo hyperpolarization transfer in a clinical MRI scanner1Department of Radiology & Biomedical Imaging, UCSF, San Francisco, CA, United States, 2GE Healthcare, San Francisco, CA, United States, 3Berkshire Magnetics, Berkeley, CA, United States, 4Department of Biochemistry, University of Florida, Gainesville, Gainesville, FL, United States
Synopsis
The purpose of this study was to investigate the feasibility of in vivo 13C->1H hyperpolarization transfer, which has significant potential advantages for detecting the distribution and metabolism of hyperpolarized 13C probes, in a clinical MRI scanner. A standalone pulsed 13C RF transmit channel was developed for operation in conjunction with the standard 1H channel of a clinical 3T MRI scanner. Operation of the custom pulsed 13C RF channel resulted in effective 13C->1H hyperpolarization transfer, as confirmed by the characteristic anti-phase appearance of 1H-detected, 1JCH-coupled doublets. 1H detection of HP [2-13C]lactate generated in vivo was achieved in a rat liver slice.
Introduction
While the vast majority of prior work on hyperpolarized (HP) 13C MRI has relied on direct 13C detection, shifting the hyperpolarization to nearby 1H spins offers significant potential advantages for detecting HP 13C magnetization. These include increased sensitivity and reduced imaging gradient requirements, due to the 4x higher gyromagnetic ratio of 1H. Moreover, specialized 13C receiver coils and electronics can be supplanted by standard 1H equipment, which more readily attains body noise dominance1 and is already tightly integrated with existing MRI methods.
Initial studies in preclinical systems have reported the feasibility of 13C->1H hyperpolarization transfers in vivo2,3. However, clinical scanners are not generally configured for heteronuclear transfers. In this work, we have developed an external pulsed 13C RF transmit channel for operation in synchronization with water-suppressed 1H MR spectroscopic acquisition on a clinical 3T scanner. The newly developed framework was applied for 13C->1H hyperpolarization transfer experiments in 1JCH-coupled systems: 1) phantom 1H MR experiments with HP [2-13C]glycerol, and 2) in vivo 1H detection of HP [2-13C]lactate generated from injected HP [2-13C]pyruvate via LDH in rat liver.
Methods
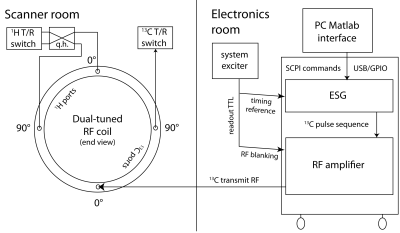
Development of an external pulsed 13C RF transmit channel- The external 13C RF architecture is illustrated in Figure 1, based on extensive modification of a commercial standalone 1H decoupler4 (GE). Commands were issued over a PC USB/GPIO connection from a custom software interface (Matlab), enabling execution of arbitrary complex RF waveforms with arbitrary timing delays with respect to a scanner TTL signal. The amplifier was also configured for RF blanking during readout intervals. 13C power was delivered into one of the two linear 13C modes of a dual-tuned 13C/1H quadrature coil, whose 1H channel was connected as usual to the 3T clinical MRI scanner. For power calibration, 13C signal was detected via the other 13C mode.
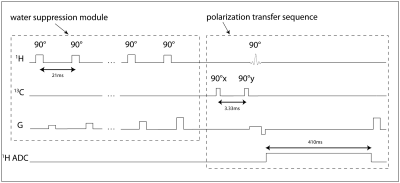
Pulse sequences- Two 13C pulse sequences were programmed-- one served for power calibration, and the other for polarization transfer. The power calibration sequence consisted of a single 500μs hard pulse. The polarization transfer sequence (Figure 2) consisted of two consecutive 500μs hard pulses with 90° RF phase shift. A custom water suppression module was programmed for the 1H MRS sequence.
MR experiments- We first directly hyperpolarized 30μL [2-13C]glycerol in a commercial Hypersense polarizer. Following dissolution in superheated D2O, 13C hyperpolarization was transferred to the proton attached to the labeled carbon. Next, the potential for 1H detection of [2-13C]lactate generated from HP [2-13C]pyruvate in vivo was tested in a normal rat. In this case, an 8mm axial 1H slice through rat liver was excited, 25s after injection of 2.5mL 80mM HP [2-13C]pyruvate. An equimolar quantity of sodium lactate (natural abundance) was co-injected with the pyruvate to stimulate HP exchange into the lactate pool.
Results
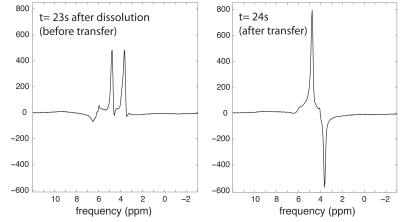
Effective hyperpolarization transfer in the [2-13C]glycerol phantom was evidenced by the characteristic anti-phase appearance of the 1H doublet on the second readout (Figure 3), following the 13C sequence. Initial 1H hyperpolarization of this doublet (in-phase) was also observed on the first readout, probably due to cross relaxation (23). About 23s was required to transport the HP sample to the MRI scanner. Since the 13C T1 relaxation time of [2-13C]glycerol was separately measured to be ~7s in D2O at 3T, we estimate that ~96% of the initial polarization was lost prior to MR data acquisition in this case. Nevertheless, the result shows clear feasibility for polarization transfer.
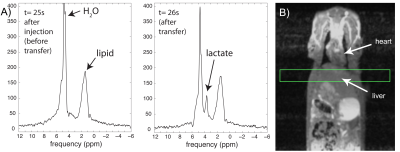
Effective transfer of hyperpolarization was also evident in the in vivo rat experiment with HP [2-13C]pyruvate. Water suppression was critical in this case, resulting in 190-fold suppression in the 8mm rat liver slice. Similar to the phantom results, a resonance corresponding to transferred HP lactate clearly appeared on the second 1H MRS readout (Figure 4), following execution of the 13C sequence.
Discussion
Our results extend into a clinical MRI scanner environment prior initial reports demonstrating the feasibility of in vivo 13C->1H hyperpolarization transfer2,3. In contrast to a recent study showing transfer from HP [1-13C]lactate to distant methyl protons3 (4.1Hz), much larger couplings (~150Hz) characterize the systems investigated here, which experience less polarization decay associated with required evolution times. Furthermore, refocusing pulses are not required since (1/JCH)<<T2*. However, the 13C T1 relaxation times of these species are also much shorter than [1-13C]lactate as a result of dipolar coupling, and additional sequence modifications are required to obtain optimized in-phase results.
Future work will focus on these optimizations, in addition to improving the effectiveness of water suppression, likely utilizing a combination of RF and gradient-enhanced approaches. We will also aim to investigate the potential for dynamic 1H imaging of hyperpolarization transferred from 13C.
Acknowledgements
This work was supported by NIH K01DK099451, R01DK105346, and P41EB013598.References
1. Edelstein WA, Glover GH, Hardy CJ, Redington RW. The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med 1986;3:604–618.
2. Mishkovsky M, Cheng T, Comment A, Gruetter R. Localized in vivo hyperpolarization transfer sequences. Magn Reson Med 2012;68:349–352. doi: 10.1002/mrm.23231.
3. Wang J, Kreis F, Wright AJ, Hesketh RL, Levitt MH, Brindle KM. Dynamic (1) H imaging of hyperpolarized [1-(13) C]lactate in vivo using a reverse INEPT experiment. Magn Reson Med 2017;137:6418. doi: 10.1002/mrm.26725.
4. von Morze C, Tropp J, Chen AP, Marco-Rius I, Van Criekinge M, Skloss TW, Mammoli D, Kurhanewicz J, Vigneron DB, Ohliger MA, Merritt ME. Sensitivity enhancement for detection of hyperpolarized 13C MRI probes with 1H spin coupling introduced by enzymatic transformation in vivo. Magn Reson Med. In press.
Figures