3058
Real-time ex-vivo measurement of brain metabolism using hyperpolarized [1-¹³C]pyruvateTalia Harris1, Assad Azar1, Gal Sapir1, Ayelet Gamliel1, Atara Nardi-Schreiber1, Jacob Sosna1, J. Moshe Gomori1, and Rachel Katz-Brull1
1Hadassah-Hebrew University Medical Center, Jerusalem, Israel
Synopsis
Translating the hyperpolarized signal observed in the brain in vivo to cerebral metabolic rates is not straightforward, as the observed signals reflect also the influx of metabolites produced in the body, the cerebral blood volume and flow, and the rate of transport across the blood brain barrier. We introduce a robust method to study rapid metabolism of hyperpolarized substrates ex vivo in viable rat brain slices and demonstrate its ability to characterize rates of LDH and PDH activities. Despite variations in these measured rates, we saw that the Lactate to Bicarbonate ratio is highly reproducible across all samples.
Introduction
The development of the dissolution dynamic nuclear polarization (dDNP) methodology by Ardenkjaer-Larsen et al.1 that can enhance the liquid state 13C NMR signal by four orders of magnitude has enabled a re-examination of cerebral MRS using hyperpolarized 13C-labeled substrates in vivo 2-10. The metabolic phenotype observed in these studies may be convoluted by significant influx of metabolic products from other organs to the brain during the hyperpolarization acquisition window and by transport of metabolites across the blood brain barrier (BBB). For these reasons we were interested in developing a system to investigate the intrinsic metabolic properties of brain tissues using hyperpolarized substrates without the convoluting effects present in vivo. Previously developed hyperpolarized perfusion-injection bioreactors11,12 cannot be used to study brain metabolism as growing cultured neuronal cells to the cell densities necessary for such experiments is difficult and, further, these cultured cells poorly represent the cellular heterogeneity and the complexity of the brain tissue in vivo13. We have adapted a perfusion system designed for maintenance of viability in ex vivo brain slices thus preserving the in vivo architecture of the brain and allowing investigation of metabolism therein. In such a system the metabolic rates observed will not be affected by influx of metabolites from the periphery or the BBB transport 14-18. The system allows rapid and repeated injections of hyperpolarized solutions. Using this perfusion-injection system we have characterized the cerebral metabolism of [1-13C]pyruvate in real time.Material and Methods
13C NMR spectra were acquired using a 5.8T high-resolution spectrometer (RS2D). Dissolution dynamic nuclear polarization (dDNP) was performed using a spin polarizer (HyperSense). 13C spectra were acquired with a 10-20° nutation angle and repetition time of 5 s. All experiments were performed on 500µm slices of the entire rat cerebrum perfused with artificial cerebral spinal fluid (aCSF) at 36°C. Kinetic data were fit assuming constant metabolic rate and T1 heterogeneity as described previously19. An additional time shift factor was introduced to account for sample settling.Results
Upon injection of hyperpolarized [1-13C]pyruvate, we were able to clearly observe the appearance [1-13C]lactate and [13C]bicarbonate. (Figure 1) In some experiments a very small signal of [1-13C]alanine was observed as well (Figure 1B). In all 16 experiments we have observed the production of hyperpolarized lactate, however, only in 13 experiments there was sufficient signal-to-noise ratio to characterize the dynamics of the [1-13C]lactate signal. For 8 of these 13 experiments there was also sufficient signal-to-noise to characterize the dynamics of the [13C]bicarbonate signal. For the remaining 5 experiments the [13C]bicarbonate signal could be observed but not analyzed kinetically. A typical experimental time course in which both [1-13C]lactate and [13C]bicarbonate signals could be fit to the kinetic model described above is shown in Figure 2. Additionally, the longitudinal relaxation constants for the different species were fit, resulting in T1,pyruvate=55±4s (n=13), T1,lactate=27±4s (n=13) and T1,bicarbonate=15±6s (n=8). Assuming 0.5 mL of hyperpolarized solution in the coil, while the remaining volume of approximately 0.875 mL is occupied by brain slices metabolic rates in the range of 1.3 – 8.3 nmol/s were observed for the production of lactate, and rates in the range of 0.42 – 0.76 nmol/s were observed for the production of bicarbonate. Despite the variation in the metabolic rates, we observed a strong correlation between the signals of [1-13C]lactate and [13C]bicarbonate. Plotting the lactate-to-pyruvate ratio versus the bicarbonate-to-pyruvate ratio for a summed spectra spanning 20 – 65 s after injection of hyperpolarized pyruvate for all of the 13 experiments for which bicarbonate signal could be observed, it can be appreciated that the [1-13C]lactate signal is ≈14 fold higher than that of [13C]bicarbonate (Figure 3).Discussion
We have established the ability of our system to characterize the enzyme activities of lactate dehydrogenase and pyruvate dehydrogenase complex in ex vivo brain slices using hyperpolarized [1-13C]pyruvate. Although there was variation in the absolute rates of lactate and bicarbonate production, presumably due to differences in the amount of viable brain tissue present in the probe, the ratio of [1-13C]lactate to [13C]bicarbonate production is highly reproducible and appears to be an inherent property of the perfused brain tissue slices in this model system.Conclusion
We have demonstrated a system to characterize the metabolism of brain tissue ex vivo using hyperpolarized substrates without the convoluting effects that complicate such interpretation in vivo. Further work is under way to study the effect of different conditions and therapeutic treatments on metabolic rates in order to better understand cerebral metabolism as well as to aid in planning and interpretation of such hyperpolarized studies in vivo.Acknowledgements
This study was supported by the European Research Council (ERC award number 338040 to RKB).References
- Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U S A 100, 10158-10163 (2003)
- Mishkovsky, M. & Comment, A. Hyperpolarized MRS: New tool to study real-time brain function and metabolism. Anal. Biochem.(2016)
- Chavarria, L., Romero-Gimenez, J., Monteagudo, E., Lope-Piedrafita, S. & Cordoba, J. Real-time assessment of 13C metabolism reveals an early lactate increase in the brain of rats with acute liver failure. NMR Biomed. 28, 17-23, (2015).
- Josan, S. et al. Effects of isoflurane anesthesia on hyperpolarized 13C metabolic measurements in rat brain. Magn. Reson. Med. 70, 1117-1124,(2013).
- Butt, S. A. et al. Imaging cerebral 2-ketoisocaproate metabolism with hyperpolarized 13C magnetic resonance spectroscopic imaging. J. Cereb. Blood. Flow Metab. 32, 1508-1514, (2012).
- Mayer, D. et al. Dynamic and high-resolution metabolic imaging of hyperpolarized [1-13C]-pyruvate in the rat brain using a high-performance gradient insert. Magn. Reson. Med. 65, 1228-1233, (2011).
- Marjańska, M. et al. In vivo 13C spectroscopy in the rat brain using hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate. J. Magn. Reson. 206, 210-218, (2010).
- Hurd, R. E. et al. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magn. Reson. Med. 63, 1137-1143, (2010).
- Hurd, R. E. et al. Cerebral dynamics and metabolism of hyperpolarized [1-13C]pyruvate using time-resolved MR spectroscopic imaging. J. Cereb. Blood Flow Metab. 30, 1734-1741, (2010)
- DeVience, S. J. et al. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-13C] pyruvate. Sci. Reports 7 (2017)
- Keshari, K. R. et al. Hyperpolarized C-13 spectroscopy and an NMR-compatible bioreactor system for the investigation of real-time cellular metabolism. Magn. Reson. Med. 63, 322-329, (2010).
- Keshari, K. R. et al. Hyperpolarized (13)C spectroscopy and an NMR-compatible bioreactor system for the investigation of real-time cellular metabolism. Magn Reson Med 63, 322-329, (2010).
- Harris, T., Eliyahu, G., Frydman, L. & Degani, H. Kinetics of hyperpolarized C-13(1)-pyruvate transport and metabolism in living human breast cancer cells. Proc. Natl. Acad. Sci. U.S.A 106, 18131-18136, (2009)
- Katz-Brull, R., Koudinov, A. R. & Degani, H. Direct detection of brain acetylcholine synthesis by magnetic resonance spectroscopy. Brain Res. 1048, 202-210 (2005)
- Lipton, P. & Whittingham, T. S. in Brain Slices (ed Raymond Dingledine) 113-153 (Springer US, 1984)
- Okada, Y. & Lipton, P. in Handbook of neurochemistry and molecular neurobiology: Brain energetics. Integration of molecular and cellular processes (eds Abel Lajtha, Gary E. Gibson, & Gerald A. Dienel) 17-39 (Springer US, 2007)
- Pardridge, W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol. Rev. 63, 1481-1535 (1983)
- Hertz, L. Metabolic studies in brain slices - past, present, and future. Front. Pharmacol. 3, (2012).
- Allouche-Arnon, H. et al. Quantification of rate constants for successive enzymatic reactions with DNP hyperpolarized MR. NMR Biomed 27, 656-662 (2014).
Figures
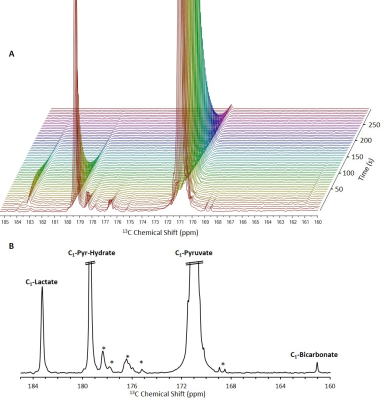
13C NMR spectra of brain slices in the
presence of 14 mM hyperpolarized [1-13C]pyruvate.
A, Stacked 13C
NMR spectra acquired with a 10° nutation
angle and a repetition time of 5 s (10 Hz line broadening). B, A 13C
NMR spectrum showing the sum of 12 spectra acquired between 15 and 70 s from
the start of dissolution. In both panels, the signals of [1-13C]pyruvate
and [1-13C]pyruvate hydrate are truncated to allow dynamic range for
visibility of the metabolite signals.
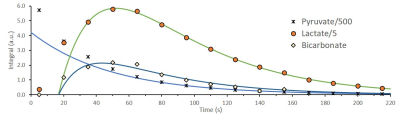
The integrated intensities of [1-13C]pyruvate,
[1-13C]lactate, and [13C]bicarbonate and their fits to
the kinetic model after injection of 14mM of hyperpolarized [1-13C]pyruvate
to viable perfused rat brain slices. To discount the effect of tissue
displacement and variation in SNR the following time points were fit for all experiment:
[1-13C]pyruvate signal was
fit from 110-220s, [1-13C]lactate signal was fit from 35-220s and [13C]bicarbonate
signal was fit from 35-140s. Assuming 0.5mL
of the coil volume is not occupied by the brain tissue, the corresponding rates
for lactate and bicarbonate production are 7.3nmol/s and 0.7nmol/s,
respectively (R2pyruvate = 0.99971, R2lactate=0.99939,
R2bicarbonate=0.95917).
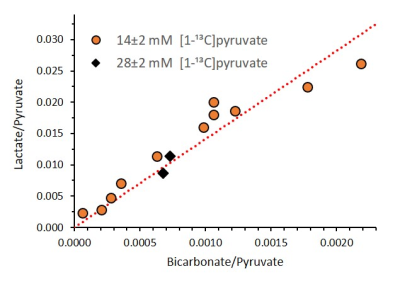
Correlation between the [1-13C]pyruvate normalized
signals of [1-13C]lactate and [13C]bicarbonate from a sum
of spectra recorded between 20 – 65 s for 13 injections from 11 different
animals on different experimental days. The linear fit was performed for the 11
experiments with 14 ± 2 mM [1-13C]pyruvate. It can be seen that
similar results were obtained for the two injections done with 28 ± 2 mM [1-13C]pyruvate.
Three injections were excluded from this graph because the [13C]bicarbonate
signal was not detectable. The resulting slope of the linear fit was 14.11 (R2=0.88).