3057
Impurities of [1-¹³C]pyruvic acid and their potential effects on the interpretation of hyperpolarized pyruvate metabolism studies1Hadassah-Hebrew University Medical Center, Jerusalem, Israel
Synopsis
Commercially available [1-13C]pyruvic acid contains impurities that have chemical shifts similar to pyruvate’s metabolic products. We show that these observed impurity peaks possess long T1s and for several peaks the chemical shift is very sensitive to the pH in the narrow physiological range measured. We concluded that in order to reliably identify low concentration metabolic products of hyperpolarized pyruvate it is crucial to characterize in situ the pH dependent impurity spectrum of the batch of [1-13C]pyruvic acid used.
Introduction
[1-13C]pyruvic acid is a leading molecular probe for dissolution dynamic nuclear polarization (dDNP) driven hyperpolarized magnetic resonance spectroscopy1. When observing metabolic products of hyperpolarized [1-13C]pyruvate whose concentration is two to three orders of magnitude lower than that of pyruvate, minor impurities of the [1-13C]pyruvic acid formulation will be of similar magnitude to the product peaks. If the chemical shift of these impurities is similar to the chemical shift of metabolic products of pyruvate care should be taken not to inadvertently confuse their signals with metabolic products. Although some of these impurities have been mentioned in other studies2-5 until now the hyperpolarized spectrum of pyruvic acid impurities has not been characterized. In this paper we compared the hyperpolarized impurities spectrum in batches of [1-13C]pyruvic acid purchased from the two main suppliers and characterized both impurities spectra in terms of longitudinal relaxation time constants and dependence of chemical shifts on pH.Materials and Methods
The OXO63 radical (GE Healthcare, UK) was obtained from Oxford Instruments Molecular Biotools (Oxford, UK). [1-13C]pyruvic acid was purchased from Sigma-Aldrich (SA, Rehovot, Israel) and from Cambridge Isotope Laboratories (CIL, Tewksbury, MA, USA). 13C NMR spectra were acquired using a 5.8 T high-resolution spectrometer (RS2D, Mundolsheim, France). Dissolution dynamic nuclear polarization (dDNP) was performed using a spin polarizer (HyperSense, Oxford instruments, Oxford, UK). Spectral processing and calculation of integrated intensities was performed using MNova (Mestrelab Research, Santiago de Compostela, Spain) or with DMFIT 6. Determination of the T1 of the hyperpolarized sites was performed by curve fitting using Matlab (Mathworks, Natick, MA, USA).Results
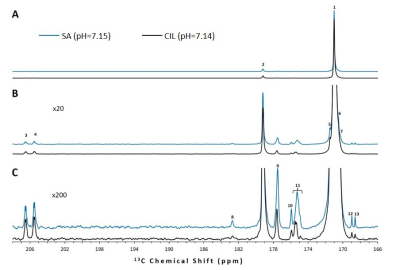
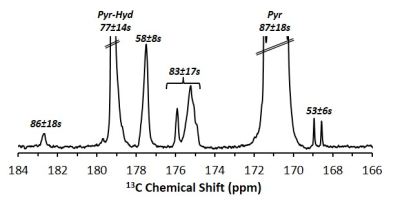
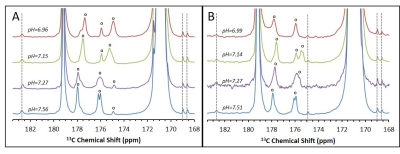
Upon dissolution of hyperpolarized [1-13C]pyruvic acid in phosphate buffer to a final concentration of 14 ± 1 mM (pH = 7.14 - 7.15) in addition to the peaks of [1-13C]pyruvate and its satellite peaks (171.0 ppm), its equilibrating hydrate (179.2), and the doublet of [2-13C]pyruvate (206.0 ppm) multiple additional impurity peaks can be observed in the formulations from both manufacturers (Figure 1) We found that all of the observed impurities have T1s above 50 s at the experimental conditions observed, with the longest T1 determined to be 92 s (Figure 2). It can be seen in the SA and CIL formulation that even in the very narrow physiological range observed (pH = 6.96 - 7.51), while several impurity peaks are insensitive to pH changes (Figure 3, peaks marked with dotted lines) other impurity peaks appear very sensitive, displaying more than 1 ppm changes in chemical shift (Figure 3, peaks marked with open circles).Discussion
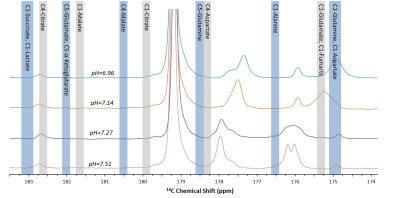
It can be appreciated that the chemical shifts of many of the sites that may be labeled upon metabolism of [1-13C]pyruvate fall near or on the chemical shifts of the impurities present in [1-13C]pyruvic acid at physiological pHs (Figure 4). Due to the long T1s of all of the impurities that we observed in the relevant chemical shift range, this problem is expected to persist throughout the experimental window. Further, as the chemical shifts of multiple sites are sensitive to slight changes in pH in the physiological range, a single reference spectra to characterize the impurities in each batch of pyruvic acid will not be sufficient.Conclusion
The non-negligible impurities present in [1-13C]pyruvic acid can lead to misinterpretation of hyperpolarized metabolic data for products whose chemical shifts is similar to the chemical shift of the impurities. Based on these results, it is crucial to characterize in situ the impurities spectra of the batch of [1-13C]pyruvic acid used for the range of pHs in the study, both in terms of chemical shift and T1, to be sure that impurities are not inadvertently identified as metabolic products of hyperpolarized pyruvate.
Acknowledgements
This work was funded by the European Research Council (Award Number 338040 to RKB).References
- Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 100, 10158-10163 (2003).
- Merritt, M. E., Harrison, C., Sherry, A. D., Malloy, C. R. & Burgess, S. C. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc Natl Acad Sci U S A 108, 19084-19089 (2011).
- Marjanska, M. et al. In vivo 13C spectroscopy in the rat brain using hyperpolarized [1-(13)C]pyruvate and [2-(13)C]pyruvate. J Magn Reson 206, 210-218, (2010).
- Pullinger, B. et al. Metabolism of hyperpolarized [1-(1)(3)C]pyruvate in the isolated perfused rat lung - an ischemia study. NMR Biomed 25, 1113-1118, (2012).
- Düwel, S. et al. Imaging of pH in vivo using hyperpolarized 13C-labelled zymonic acid. Nature Communications 8, 15126 (2017).
- Massiot, D. et al. Modelling one- and two-dimensional solid-state NMR spectra. Magnet. Reson. Chem. 40, 70-76, (2002).
- Wishart, D. S. et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res 41, D801-807, (2013).
Figures