3055
Simple and fast hyperpolarization of a biomolecule: Theory and ExperimentStephan Berner1,2,3, Stephan Knecht1,4, Andreas Benjamin Schmidt1,4, Mirko Zimmermann1, Jürgen Hennig1, Dominik von Elverfeldt1, and Jan-Bernd Hövener4
1Department of Radiology, Medical Physics, Medical Center—University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2DKTK, Freiburg, Germany, 3DKFZ, Heidelberg, Germany, 4Department of Radiology and Neuroradiology, Section Biomedical Imaging, MOIN CC University Medical Center Schleswig-Holstein, University of Kiel, Kiel, Germany
Synopsis
Hyperpolarization overcomes the biggest limitation of MRI: its low sensitivity, and enables metabolite mapping. Hyperpolarized 13C magnetization can be produced by transferring the spin order of parahydrogen into 13C by hydrogenation followed by a sequence of 1H and 13C pulses. However, it is possible to hyperpolarize AA’X spin systems by two pulses on 13C. Theoretical models were developed to describe the polarization transfer and significant signal increase was observed for the biomolecule succinate after spin order transfer directly in the magnet of a commercial MRI system. The experimental data is well described by theoretical calculations except for an overall scaling factor.
Introduction
Magnetic Resonance Imaging (MRI) is a powerful tool in clinical diagnosis despite its low sensitivity that is caused by the low thermal polarization of the order of 10-5. For many (spectroscopic) applications the signal is simply too low. This issue is partially resolved by hyperpolarization methods, whose signal enhancement enables the monitoring of metabolism. Spin Order Transfer (SOT) sequences were developed to transform spin order from parahydrogen into 13C magnetization by appropriate radiofrequency pulses on 1H and 13C, free evolution intervals, and heteronuclear decoupling schemes during hydrogenation. However, it is possible to hyperpolarize an AA’X spin system with a much simpler experiment by applying a single 90° 13C pulse followed by a refocusing pulse directly after hydrogenation without the need for decoupling [1]. Here we present a theoretical model and first experimental data of a biologically relevant molecule obtained in a preclinical MR system.Methods
1-13C,2,3-2H2-Succinate was formed by catalytic hydrogenation of fumarate under PASADENA [2] conditions and polarized by means of SAMBADENA [3] in the magnet bore of a preclinical 7T MR system (Bruker Biospec, Germany). The pulse sequence 90x° – ts/2 – 180° – ts/2 – acquisition (free evolution time ts) was applied after a time duration thyd after the onset of the hydrogenation. The parameters ts and thyd were experimentally optimized, a mathematical model was developed [3] and fitted to the data points. Quantum mechanical simulations were carried out to predict the theoretical polarization yield as a function of ts. The process of hydrogenation was considered by averaging the density matrix over thyd. The resulting density matrix was manipulated by radio frequency pulses and evolved in time under the Hamiltonian of an AA’X spin system. Relaxation effects were neglected. Additionally, the effect of erroneous flip angles on the polarization yield was calculated. The experimental polarization yield was quantified by comparison with a thermally polarized reference, assuming a parahydrogen fraction of 100% and complete hydrogenation.Results
The highest theoretical polarization yield was PTheory = 48% for ts = 68 ms. The greatest signal enhancement observed experimentally was 1400-fold, corresponding to a polarization level of ≈ 0.83% for ts = 60 ms and 70 ms. The calculated polarization yield as function of ts was well reproduced experimentally, except for a constant scaling factor of 57.83 (Fig. 1). The optimal hydrogenation duration thyd was found to be between 3 s and 6 s (Fig. 2). By fitting a kinetic model to the data points, the relaxation constant of the para order Tpara and the hydrogenation constant Tcat were determined to Tpara = (13 ± 5) s and Tcat =(1.4 ± 0.9) s.Discussion
In only a few seconds, a significant 13C-signal increase was experimentally achieved in the bore of an MR system, although with an amplitude of approximately 58-times less than predicted which is too little for biomedical application. The missing signal may be attributed to relaxation effects, experimental errors and incomplete hydrogenation. For deviations of 50% of the nominal flip angle, the theoretical polarization yield is still greater than 25%. Note that polarization levels of about 7% were achieved by means of Goldman’s sequence [4] (theoretical polarization: 99%) using the same setup [5].Conclusion
In comparison to other transfer methods, the treated two-pulse sequence is much simpler, faster, and more robust against erroneous flip angles and off resonant pulses. However, the high theoretically predicted polarization yields of about 50% could, so far, not be achieved experimentally. The low experimentally observed signal enhancements may suggest either experimental errors or a significant effect that is not yet considered in the theoretical model. Note that this sequence is of interest for MR systems that cannot play out either pulses on two channels or demanding decoupling sequences.Acknowledgements
Support by the DFG (HO 4604/1-1 and HO 4604/2-1), the DKFZ/DKTK and the Heinrich-Böll-Stiftung (ABS, P131623) and the contribution of the COST Action TD1103 is greatly acknowledged.References
- Haake, M., Natterer, J. & Bargon, J. Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei. J. Am. Chem. Soc. 118, 8688–8691 (1996)
- Bowers, C. R. & Weitekamp, D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987).
- Schmidt, A. B. et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat. Commun. 8, ncomms14535 (2017).
- Goldman, M. & Jóhannesson, H. Conversion of a proton pair para order into 13C polarization by rf irradiation, for use in MRI. Comptes Rendus Phys. 6, 575–581 (2005).
- Stephan Berner, Andreas Schmidt, Schimpf Waldemar & Jan-Bernd Hövener. Hyperpolarization of a biomolecule to 7% in an MRI using SAMBADENA. Proceedings of EMIM 2017
Figures
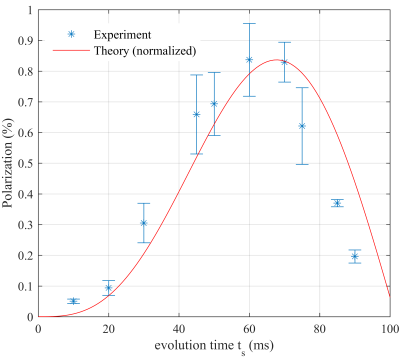
Fig. 1: Experimental (stars) and theoretical (line) polarization
yield as a function of the evolution time ts of the pulse sequence. The theoretical polarization
yield was scaled to match the experimental data. Each data point was measured 3
times and the error bars represent the standard deviation.
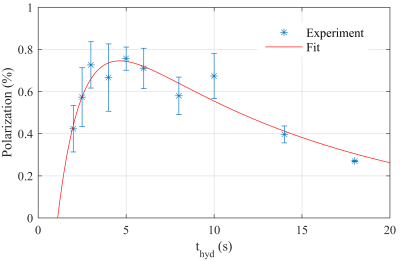
Fig. 2: 13C-polarization yield as a function of the hydrogenation time thyd
(stars) and fit (line, Tpara = (13 ± 5) s and Tcat =(1.4
± 0.9) s).