3052
Application of a novel 13C hyperpolarized metabolic tracer for γ-Glutamyl transferase activity in vivo tumor xenograft1Radiation Biology Branch, CCR, NCI, NIH, Bethesda, MD, United States, 2Department of Chemistry and Biotechnology, Graduate School of Engineering, UT, Bunkyo-ku, Tokyo, Japan, 3Department of Molecular Imaging & Theranostics, QST, Chiba-shi, Japan, 4Electrical Engineering, Department of Electrical Engineering, DTU, Lyngby, Denmark, 5Institute of Physics of Biological Systems, EPFL, Lausanne, Swaziland
Synopsis
This research aimed to develop the non-invasive in vivo detection of γ-glutamyl transferase (GGT) activity by a novel GGT molecular probe, γ-Glu-[1-13C]Gly, in combination with hyperpolarized (HP) 13C Magnetic Resonance (MR) spectroscopy. We succeed in detecting the strong HP 13C MR signal of γ-Glu-[1-13C]Gly from tumor xenograft in vivo. Detecting HP 13C MR signal from the metabolite of this novel probe in tumor xenograft is our next challenge.
[Purpose]:
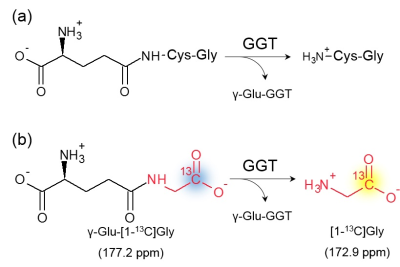
γ-Glutamyl transferase (GGT) is membrane-bound enzyme that catalyzes the cleavage of γ-glutamyl moiety of extracellular glutathione to supply cysteine for intracellular synthesis of glutathione as anti-oxidant (Figure 1a).1 Elevated GGT activity has been found in wide range of cancers, where GGT promotes tumor cell survival, tumor progression, and resistance to radiotherapy and chemotherapies.2, 3 Based on biological and clinical studies, we hypothesize that non-invasive methods for real-time monitoring of GGT activity will provide the information of cancer characterization, prognosis, and development of therapeutic strategy.
Hyperpolarized (HP) 13C Magnetic Resonance spectroscopy (MRS) and imaging which have been newly developed modality enable non-invasive molecular imaging of real-time metabolism in tumor.4 Despite of the advantageous feature, only a limited number of 13C-labeled probes have been utilized for in vivo applications because of their physicochemical property. Dr. Nishihara et al., however, have successfully designed and prepared a novel HP molecular probe for GGT activity, γ-Glu-[1-13C]Gly (Figure 1 b).5 γ-Glu-[1-13C]Gly was designed to produce a distinct 13C signal in chemical shift range by the enzymatic cleavage, due to the positively charged amino group of released [1-13C]Gly in physiological pH. It was demonstrated that this probe can monitor real-time GGT activity in vivo rat kidney as well as in vitro homogenate samples.5
Herein, we investigated to apply this novel probe with HP-MRS technology to in vivo tumor xenograft.
[Methods]:
GGT probe: The GGT probe, Glu-[1-13C]Gly was obtained from Dr. Sando group in University of Tokyo by agreement.
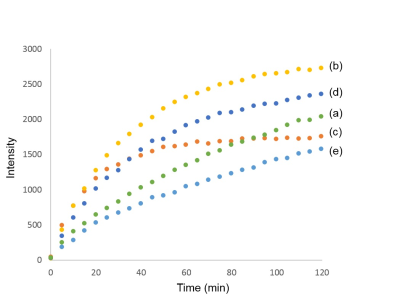
Optimization of the probe polarization: γ-Glu-[1-13C]Gly (3.8 M) dissolved in a NaOH 5.0 M solution was hyperpolarized with various concentration of OX063 (17.3 mM, 26.0 mM, or 34.6 mM) and gadolinium chelate (Gd) (2.5 mM or 7.5 mM), and each of polarization build-up was monitored up to 2 hours. Conventional recipe was polarized with OX063 17.3 mM and Gd 2.5 mM as a control.5
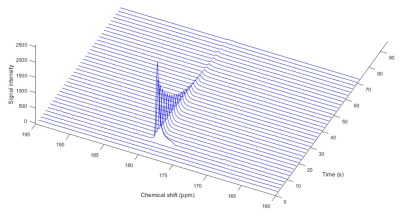
T1 measurement of γ-Glu-[1-13C]Gly at dissolved state: γ-Glu-[1-13C]Gly (3.8 M) dissolved in a NaOH (5.0 M) solution containing OX063 26.0 mM and Gd 2.5 mM was polarized and dissolved with superheated buffer (Tris 50 mM, EDTA 0.27 mM, HCl 55 mM). 2.0 mL of HP solution was injected in a vial containing 2.0 mL of PBS, and the 13C MR spectra were acquired every 2 seconds with a spectral width of 2,500 Hz, repetition time of 2,000 ms and flip angle of 5 degrees.
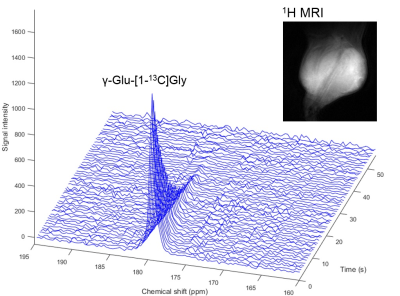
Hyperpolarized 13C-MRS in vivo study: γ-Glu-[1-13C]Gly ( 3.8 M in NaOH 5.0 M) 40 μL, containing 26.0 mM OX063 and 2.5 mM gadolinium chelate, was hyperpolarized up to 1.5 hours. The hyperpolarized sample was rapidly dissolved in 4.0 mL of a superheated buffer (Tris 50 mM, EDTA 0.27 mM, HCl 55 mM), and dissolved solution was intravenously injected (12 μL/g body weight). Hyperpolarized 13C MRI studies were performed on a 3T scanner using a 17 mm home-built 13C solenoid coil placed inside of a saddle coil for 1H. The 13C MR spectra were acquired every 3 seconds with a spectral width of 2,500 Hz, repetition time of 3000 ms and flip angle of 30 degrees. In vivo application was performed in a mouse harboring a OVCAR-5 tumor.
[Results]:
Solubility of γ-Glu-[1-13C]Gly was improved up to 3.8 M with 5M NaOH compared to the 2.8 M with conventional recipe, and this new formulation was confirmed to become glassy state at cryogenic temperature without glycerol. The result of build-up showed that the formulation of γ-Glu-[1-13C]Gly (3.8 M) with 26.0 mM OX063 and 2.5 mM Gd has the highest efficiency at time point of 60 minutes (Figure. 2).
Hyperpolarized γ-Glu-[1-13C]Gly with 26.0 mM OX063 and 2.5 mM Gd was dissolved and measured by time resolved single-scan 13C NMR spectra and 13C probe signal was detected at 179.7 ppm (Figure. 3). T1 was calculated to be 26.9 sec from the decay curve of 13C signal, confirming that 1.5-fold higher concentration of OX063 in the formulation did not affect shorting T1 of γ-Glu-[1-13C]Gly.5
13C dynamic spectra of γ-Glu-[1-13C]Gly injected in xenograft mouse was shown in Figure 4. Signal-to-noise ratio of the γ-Glu-[1-13C]Gly peak was good, however, [1-13C]Gly as metabolite signal via GGT enzymatic reaction was not seen as strong enough to be kinetically analyzed.
[Conclusion]:
We successfully detected 13C signal of γ-Glu-[1-13C]Gly as a parent GGT probe in vivo tumor xenograft. To monitor its metabolite peak in vivo, optimized flip angle for excitation pulse or signal denoising of post-processing will be applied and we will show the results in the presentation.Acknowledgements
No acknowledgement found.References
1. Grimm C., et al., Association of gamma-Glutamyl Transferase with Severity of Disease at Diagnosis and Prognosis of Ovarian Cancer. BR. J. Cancer, 2013, 109:610–614.
2. Waxman J. D., Glutathione S-Transferases: Role in Alkylating Agent Resistance and Possible Target for Modulation Chemotherapy— A Review. Cancer Res., 1990, 50, 6449-6454.
3. Cole S.P., Multidrug Resistance Protein 1 (MRP1, ABCC1), a "Multitasking" ATP-Binding Cassette (ABC) Transporter. J. Biol. Chem., 2014, 289(45): 30880–30888.
4. Saito K., et al., 13C-MR Spectroscopic Imaging with Hyperpolarized [1-13C]Pyruvate Detects Early Response to Radiotherapy in SCC Tumors and HT-29 Tumors. Clin. Cancer Res, 2015, 21(22):5073-5081
5. Nishihara T. et al., Direct Monitoring of γ-Glutamyl Transpeptidase Activity In Vivo Using a Hyperpolarized 13C-Labeled Molecular Probe. Angew. Chem. Int. Ed., 2016, 55(36):10626-10629
Figures