3051
A Carbon-Fibre Sheet Resistor for MR, CT, SPECT and PET-compatible Temperature Maintenance in Small Animals1University of Oxford, CRUK/MRC Oxford Institute for Radiation Oncology, Oxford, United Kingdom
Synopsis
A resistive heater that is compatible with MR, CT, SPECT and PET imaging has been produced from a commercially available carbon-fibre sheet. Adequacy of temperature maintenance and insensitivity of MR and CT imaging to the presence and use of the heater is shown. Multimodal MR-CT-PET-SPECT imaging of the lower abdomen is demonstrated in vivo in the physiologically maintained and viable anaesthetised mouse.
INTRODUCTION
Active heating is required to avoid hypothermia in anaesthetised animals. Small resistive MR-compatible heaters1,2 are useful where space limitations prevent the use of circulating fluids but CT imaging is severely compromised by these, due to the presence of high atomic number elements. PET and SPECT imaging are unaffected by these heaters unless a corrupted CT scan is used for attenuation correction. We describe a carbon-fibre sheet heater element, used in conjunction with 100 kHz alternating current (AC) that is demonstrated to be MR, CT, SPECT and PET-compatible.METHODS
Cradles that were size-matched to the RF coils used were CAD-drawn (Solidworks) and printed using ABS plastic (HP Designjet 3D). A 50 Ω resistive heater element formed from 0.75 mm thick carbon-fibre sheet (RS, 764-8700) cut into a U-shape with 2x7 mm wide legs, each 130 mm long and spaced by 1.5 mm, was glued to the underside of the cradles as shown in Fig. 1. 100 kHz AC at 1-2 W was applied using a power amplifier (Pioneer A-20) driven by a Pierce oscillator providing a cradle base temperature of ca. 37° C.
MRI was performed at 7 T using a 26 mm diameter, 100 mm long RF coil for system validation purposes, and with a 32 mm diameter, 45 mm long RF coil for multimodal imaging. MR-compatibility of the heater element was tested using global pulse-acquire spectroscopy, whole-body respiratory-gated bSSFP and FLASH imaging each with the heater turned on and off. For multi-modal imaging respiratory-gated FLASH MRI was performed repeatedly to effect DCE-MRI.
CT, PET and SPECT imaging were performed in a single gantry system (Vector4CT, MILabs) immediately before MRI, on animals that were held in the same cradles as for MRI. CT–compatibility was tested in vivo in the absence of any heater, and in the presence of the copper wire or carbon-fibre sheet heater elements. Hounsfield Units were measured to assess the impact of heater element composition on x-ray attenuation.
For validation of thermal stability and MR-compatibility, normal mice (n=3) were anaesthetised with isoflurane (1-4%) in oxygen-enriched air and placed on the imaging cradle where respiration rate was maintained at 40-60 breaths/minute. Rectal temperature was monitored for 50 minutes before animals were recovered. Homeothermic control was effected through manual switching of the current as the target temperature threshold was crossed.
Multimodal MR-CT-PET-SPECT imaging of kidneys was performed using anaesthesia and physiological maintenance as above, using [99mTc]-mercaptoacetyltriglycine (SPECT), [18F]-fluorodeoxyglucose (PET), anatomical CT, and DCE-MRI. Images were aligned and overlaid using rigid-body registration. Further MR-CT-PET imaging has been performed successfully using this heating system in 55 mice undergoing study for other purposes.
RESULTS
Core temperature was maintained using carbon-fibre sheet heating at 36°C±0.9°C for 50 minutes and animals recovered from anaesthesia quickly.
NMR spectra, Fig. 2A, showed no lineshape distortions or frequency shift attributable to current flow through the heater. Similarly, no distortions attributable to the current were observed in respiratory-gated FLASH or bSSFP imaging (Figs. 2B and 2D, respectively). Residual artefacts result, primarily, from cardiac and gut motion.
Upon CT imaging the copper wire heater produced severe image streaking, Fig. 3A; organs were poorly-defined and poor estimations of CT intensities were made, Table 1. Conversely, no heater-related artefacts were observed when the carbon-fibre sheet heater was used, Fig. 3B, and absolute image quantification was equivalent to that measured in the absence of any heater, Table 1.
Multimodal PET/SPECT/CT/DCE-MRI imaging was performed and images could be easily co-registered, displayed, analysed and presented (Fig. 4).
DISCUSSION
A compact MR, CT, PET and SPECT-compatible carbon-fibre sheet resistive heating apparatus that is easy to produce and integrate in imaging cradles is described. Whilst manual control of the homeothermy was used in this report automated control is now established. MR-compatibility is achieved through temporal averaging of the effects of stray magnetic field towards zero, whilst CT-compatibility is achieved through the use of a low atomic number material. The absence of the streak-artefacts formed in CT images when even small amounts of copper are present in a heater element improved identification of organs and allowed absolute quantification of CT image intensities. Accurate CT-based attenuation correction of PET and SPECT images could be performed and these images can be registered with MR.CONCLUSION
Carbon-fibre sheet resistors powered with high frequency AC allow homeothermic maintenance that is compatible with multimodality imaging using MR, CT, PET and SPECT. The heater is small, and easy to produce and integrate into cradles for multi-modal imaging.Acknowledgements
This work was supported by Cancer Research UK (C5255/A12678 and C2522/A10339) and the Medical Research Council.References
1. Kersemans, V., Gilchrist, S., Allen, P. D., Beech, J. S., Kinchesh, P., Vojnovic, B., & Smart, S. C. (2015). A resistive heating system for homeothermic maintenance in small animals. Magnetic Resonance Imaging, 33(6), 847–851.
2. Gilchrist S, Gomes AL, Kinchesh P, Kersemans V, Allen PD, Smart SC (2016) An MRI-Compatible High Frequency AC Resistive Heating System for Homeothermic Maintenance in Small Animals. PLoS ONE 11(11): e0164920.
3. Bolus,
D., Morgan, D. & Berland, L. (2017) Effective use of the Hounsfield unit in the age of variable energy CT. Abdom Radiol 42: 766.
Figures
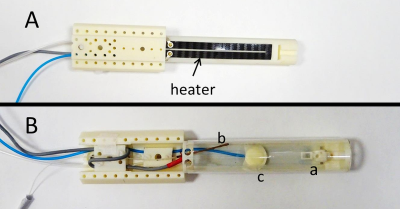
Figure 1: Carbon-fibre sheet resistive heater embedded in a 3D-printed, flat-base multimodal imaging cradle: (A) Bottom and (B) Top view.
The cradle contains (a) a mouthpiece assembly consisting of a base block, a vertical and horizontal adjustable mouth bar and an anaesthetic gas delivery tube, (b) optical fibre for temperature monitoring and (c) pressure balloon for respiration monitoring.
100 kHz AC power forms stray magnetic fields that are self-cancelling every 10 μs so cumulative MR phase accumulation and image distortions are small. CT-compatibility is achieved through the use of a low atomic number material.
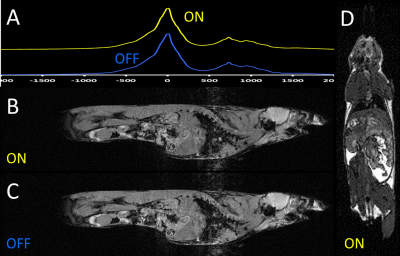
Figure 2: Impact of current flow through the carbon-fibre sheet heater element on MRI.
(A) Global pulse-acquire spectroscopy: heater turned off and on;
(B) FLASH MRI: heater turned on;
(C) FLASH MRI: heater turned off;
(D) bSSFP MRI: heater turned on.
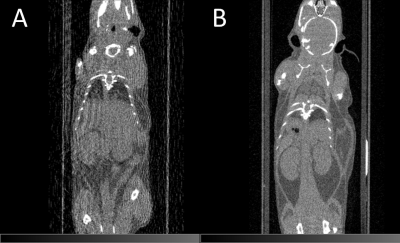
Figure 3: Impact of the heater material on CT image quality.
CT imaging using (A) the 150 μm diameter copper wire heater or (B) carbon-fibre sheet heater.
Streak artefacts due to the presence of the heater are absent when using the carbon fibre heater and image intensities remain intact.
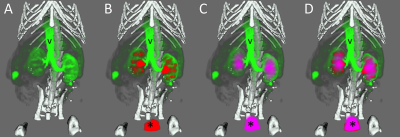
Figure 4: Multimodal imaging of a mouse using the carbon-fibre sheet resistive heater embedded in a 3D-printed, flat-base multimodal imaging cradle. The skeleton, kidneys, major vessels to the kidneys (v) and the bladder (*) are marked up.
The skeleton (white) was imaged by CT, whilst [99mTc]-mercaptoacetyltriglycine (red), [18F]-fluorodeoxyglucose (purple) and Gadodiamide (green) were used for SPECT, PET and DCE-MRI of the kidneys, respectively. Each panel shows an additional layer of the co-registered, segmented image: (A) CT + MRI, (B) CT + MRI + SPECT, (C) CT + MRI + PET, (D) CT + MRI + PET + SPECT.
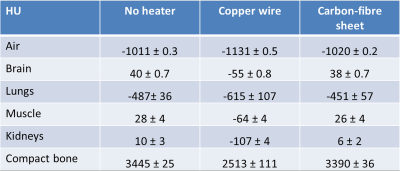
Table 1: Impact of copper wire and carbon-fibre sheet resistive heater elements on X-ray attenuation as measured in Hounsfield Units (HU).
Values are equivalent with and without the carbon-fibre sheet heater and correspond to values reported in literature. The HU scale is calibrated such that the value for water is 0 HU and that for air is −1000 HU. Measured tissue attenuation is directly dependent on the energy of the X-ray beam3.