3050
Mapping of spatial distribution of the olfactory bulb new neurons at single cell level using iron oxide assisted-MRINikorn Pothayee1, Claire Perez2, Stephen Dodd1, and Alan P. Koretsky1
1National Institutes of Health, Bethesda, MD, United States, 2University of Guam, Mangiloa, Guam
Synopsis
In this study, we aimed to develop a method that could quantitatively track new neurons in the olfactory bulb (OB). We first established that MRI signals detected in the OB were those of single labeled new neurons that migrate from the neurogenic niche into the OB. Further, we combined the anatomical MRI enhancing properties of Mn2+ to evaluate the preference of new neurons for specific layers within the OB and to determine whether sensory enrichment affects distribution of adult-born neurons within the OB layers.
Introduction
In mammalian brains, neural precursor cells originate from the subventricular zone (SVZ) and give rise to neuronal precursor cells that migrate to the olfactory bulb (OB). In the OB, these cells differentiate into new neurons, which are thought to be involved with olfactory processing and learning. Over the past decade, magnetic resonance imaging (MRI) has been demonstrated to be able to detect single cells and to track new neurons in the OB of live animals [1,2,3]. This requires labeling the precursor cells with iron oxide based contrast to give sufficient MRI signal. Neural progenitor cells in the SVZ were labeled with micron-sized iron oxide particles (MPIOs). Following their migration into the OB, the distribution of MPIO-containing new neurons in the OB were detected with high resolution MRI (Figure 1). In this study, we aimed to confirm that the MRI signals could be used to detected single new neuron in the OB and whether the distribution of new neurons could be mapped in laminar specific manner. Finally, we investigate whether odor enrichment can influence the spatial distribution of new neurons in the OB of live animals.Method
Identification of MPIO within single new neuron was analyzed on OB tissue sections. Adult male rats (n =4) 6 weeks of age received intraventricular injection of MPIOs (Bangs Laboratories, Inc., Fishers, IN) as previously described [3]. 4 weeks after injection, the animals were euthanized and perfused with 5% formalin and the OB were removed and processed for cryosection to obtained thin OB section (25 micron-thick). Total of 8 OB sections from each rat were randomly chosen and processed for ex vivo MRI and immunohistochemistry analyses (Figure 2). For in vivo experiment, a separate group of the rats (n =3) were injected with MPIOs and kept for 4 weeks. 24 prior to MR imaging, animals were infused with 37mg Mn2+/kg. Data was acquired on an 11.7 T animal MRI system (30 cm 11.7 T horizontal magnet, (Magnex Scientific, Oxford, England), MRI Electronics, Bruker Biospin, Billerica, MA, and 12 cm 3D gradients (Resonance Research Inc, Billerica, MA) using a volume transmit coil and a custom built, 1 cm diameter, receive-only surface-coil. Animals were imaged at four weeks post-injection of MPIO and were placed in a MRI compatible cradle with a stereotactic head-frame under continual anesthesia of 2% isoflurane in 75% O2/25% medical air. Animals were orally intubated and mechanically ventilated at 50-60 breaths/min while the end tidal CO2 and respiration patterns were monitored. Body temperature was maintained at 37° C using a circulating water bath. 3D Multi-gradient echo (MGE) sequences were used for MRI with the following parameters: FOV 1.28 cm x 1.44 cm x 0.96 cm, Matrix 256 x 288 x 192 (50 mm isotropic resolution), 50 kHz bandwidth, multiple TEs 4.25, 11.75, 19.25, and 26.75 ms, and TR 32 ms. Images were reconstructed using MIPAV. Images from the second and third echo were thresholded at 3 x standard deviation of the noise of the surrounding 5 pixels to select the MPIOs from the background. This thresholded mask was overlaid on the original images from the first echo. From these composite images, the MPIOs were counted in each layer of the olfactory bulbResults and Discussion
In this study, we demonstrate that MRI can be used to identify single new neurons in the rat olfactory bulb (OB) with layer specificity. The MPIO-labeled neuroblasts give a strong T2* effect and can be easily identified as hypointense spots. Moreover, signals measured from MRI were well-correlated with MPIOs (Figure 3). Immunohistological identification confirmed that the MPIOs were indeed maintained in the new neurons following their differentiation from precursor cells and had limited non-specific uptake in astrocytes and microglia (Figure 4). Therefore, the results show that MPIO-generated MRI contrast can be used to detect individual labeled new neurons and their integration within the OB. Furthermore, when combining this method with Mn-enhanced MRI (MEMRI), it could be applied to study the effect of odor enrichment on the layer specific distribution of the new neurons in the OB. The MPIO-positive cells were imaged in the olfactory bulb with the aid of Mn2+ (Figure 5). The addition of manganese was useful not only because it is excellent T1 relaxation agent, which increases the signal to noise, but also has the unique property of enhancing cytoarhitecture of the olfactory bulb [4]. The ability to map distribution of the new neurons in vivo using MRI should afford interesting new avenues for future study with regard to adult neurogenesis and experience-dependent integration of new neurons.Acknowledgements
This research was supported by the NINDS Intramural Research Program of NIH.References
[1] Shapiro, E.M., Gonzalez-Perez, O., Manuel Garcia-Verdugo et al., 2006, Neuroimage 32, 1150-1157. [2] Sumner, J.P., Shapiro, E.M., Maric, D. et al., 2009, Neuroimage 44, 671-678. [3] Pothayee N., Cummings D.M., Schoenfeld T. et al., 2017, Neuroimage, 158., 232-241. [4] Aoki, I., Wu, Y.J., Silva, A.C.et al., 2004, Neuroimage 22, 1046-1059.Figures
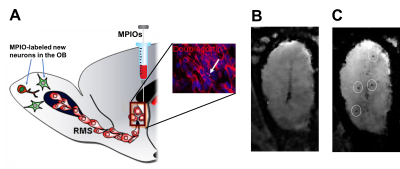
Figure
1. A) Labeling of neural precursor cells by direct injection of MPIOs into the lateral
ventricle near the SVZ which allowed for an uptake of MPIOs into neuroblasts
(doublecortin-postive) that migrate into the OB along the RMS and differentiate
into mature interneurons in the OB B) Coronal MRI image of the OB of the rat 1
week after MPIO injection. C) The same rat was imaged at 2 weeks after MPIO
injection showing migration of MPIO-induced spots (circled) toward the outer
layer of the OB.

Figure
2. The workflow to identify whether MPIO-induced hypointense signals (spots)
represent single new neurons in the OB.

Figure 3. (A) Ex vivo MRI image of OB slice. (B)
Fluorescent image converted to grayscale of the same OB slice shown in panel A.
(C) Co-registration of MRI and fluorescent images showing MPIO detection in
both MRI and fluorescent images which was well-correlated (boxed). (D)Inset of
the overlaid image. (E) Histological evaluations of the particles shown in
panel D showed that particles (green) are closely associated with nuclei(DAPI, blue) indicating
internalization by the cells.

Figure
4. A) MPIO were found in all layers of the OB including granule cell layer
(GCL), external plexiform layer (EPL), mitral cell layer (MCL), and glomerular layer
(GL) with more than 98% MPIOs were closely associated with nuclei (DAPI). B)
Immunostaining with either IBA1 or GFAP showed that MPIOs were mostly (>
90%) resided outside microglia or astrocytes. C) Immunostaining with antibodies
against tyrosince hydroxylase or calretinin showed that the MPIOs are within
the interneurons.
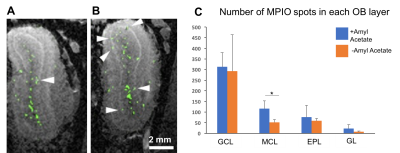
Figure
5. Combining MPIO imaging (color-coded in green) with Mn-enhanced
MRI allowed for detection of labeled cells in laminar-specific distribution. A)
MRI image of the OB from control group that did not receive amyl acetate. B)
MRI images of the OB from group that received amyacetate for 4 weeks. C) Odor-enrichment
appeared to increase of MPIO-labeled new neurons in the mitral cell layer (highlighted
by arrows in panel A and B).