3048
An Improved CEST MRI Reporter Gene for Molecular Imaging of Cell and Viral Based Therapeutics1Martinos Center for Biomedcial Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Neurosurgery, Brigham & Women's Hospital and Harvard Medical School, Boston, MA, United States, 3Department of Biomedical Engineering, Michigan State University, East Lansing, MI, United States, 4The Institute of Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, United States, 5Department of Radiology, Michigan State University, East Lansing, MI, United States
Synopsis
The ability to image cell- or viral-based therapeutics is critical for optimizing therapeutic strategies and assessing efficacy. A lysine rich protein (LRP) chemical exchange saturation transfer (CEST) MRI reporter gene has previously been developed and successfully used to image oncolytic viruses and tumor cells. However, the highly repetitive nature of the LRP reporter gene sequence lead to DNA recombination events and the expression of a range of truncated LRP protein fragments, thereby greatly limiting the CEST sensitivity. Here we report the use of a redesigned LRP reporter (rdLRP), which demonstrated excellent stability and CEST sensitivity.
Introduction
Molecular imaging of biological processes and biological therapeutics has benefited greatly from the development of reporter genes. The vast majority of such reporter genes have relied on the expression of optical imaging reporters. More recently, an MRI reporter gene was developed that was detectable by chemical exchange saturation transfer (CEST) MRI (1). The reporter gene encoded a lysine rich protein (LRP) with 200 lysine residues, where the amide exchangeable protons of lysine are in fast chemical exchange (~400 Hz) with the bulk water protons. The LRP reporter gene has been used to detect 9L rat glioma cells (1,2) as well as to image oncolytic virus infection of rat glioma models (3). However, the highly repetitive nature of the LRP DNA sequence lead to DNA recombination events and the expression of a wide range of truncated LRP protein fragments and not full length protein, thus limiting the CEST sensitivity. Here we report on a redesigned LRP (rdLRP) reporter gene that breaks up the repetitive lysine encoding sequences and demonstrated improved stability and CEST sensitivity.Methods
Five synthetic peptides (Genscript, Piscataway, NJ) were dissolved in PBS and CEST Z-spectra were obtained on 17.5 T Bruker vertical bore MRI scanner (Bruker Biospin, Billerica, MA) with imaging parameters as described previously (1,2). The rdLRP plasmid, under control of the cytomegalovirus (CMV) promoter, was transfected into HEK293T cells using lipofectamine. After 24 hours incubation, the cells were lysed in pH 7.5 RIPA buffer and underwent 3 freeze/thaw/sonication cycles. Protein content of the supernatant was measured and protein concentrations of control and LRP transfected cell lysates were adjusted to be equivalent (typically 10 µg/µl protein). Control and rdLRP cell lysate samples were loaded into 5 mm od sample tubes and placed into 50 ml Falcon tubes containing water. CEST Z-spectra were acquired on a 4.7 T Bruker MRI scanner. The Magnetization Transfer Ratio asymmetry (MTRasym), or CEST contrast, was calculated from the difference between the signal intensities acquired with negative (ω-) and positive (ω+) saturation frequency offsets from water, normalized by the signal intensity acquired with no saturation pulse.Results and Discussion
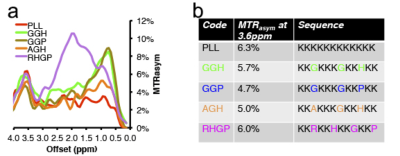
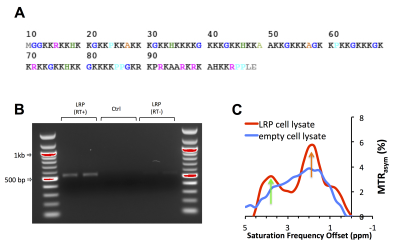
A number of different lysine polypeptides with different amino acid motifs separating lysine residues were screened to find a peptide that disrupts the repetitive lysine sequences and has optimal CEST contrast. The CEST contrast of a lysine polypeptide containing an arginine, histidine, glycine and proline (RHGP) amino acid motif was shown to have virtually the same CEST contrast as poly-L-arginine (PLL) for the amide exchangeable protons at 3.6 ppm chemical shift (Figure 1A,B). In addition, a strong CEST contrast was observed at the amine chemical shift of 1.8 ppm attributed to the guanidyl amines of the arginine residue. The amine CEST contrast may provide a further boost in the CEST sensitivity of reporters with the RHGP motif as well as provide a means for performing amine/amide ratiometric CEST imaging for obtaining quantitative pH measurements independent of reporter concentration. In addition, the very rapid exchange rate (≥1 kHz) of the amine exchangeable protons may provide a means of distinguishing the CEST reporter from endogenous proteins with slow proton chemical exchange by exploiting exchange rate specific CEST methods such as Frequency Labeled EXchange transfer (FLEX) (4). A redesigned LRP reporter (rdLRP) was designed using the RHGP motif that consisted of arginine, histidine, glycine, and proline amino acids separated by different numbers of lysine amino acids (Figure 2A). The rdLRP contains no DNA repeats or GC rich regions resulting in a significantly more stable protein with 30% less positively charged amino acids compared to the original LRP. The improved stability is evident in Northern blots using RT-PCR of total mRNA from cell lysates transfected with rdLRP, where only a single well-defined mRNA band is observed at the base pair length expected for full length rdLRP (Figure 2B). In contrast, a ladder profile of bands of different base-pair lengths was observed previously in RT-PCR of cell lysates transfected with the original LRP reporter gene (1). Similar to the RHGP peptide, a distinct increase in MTRasym is observed at the amine and amide exchangeable proton frequencies for cells transfected with the rdLRP relative to control, untransfected cells (Figure 2C).Conclusions
The redesigned LRP reporter demonstrates excellent stability with distinct CEST contrast observed for both amide and guanidyl amine exchangeable protons of the redesigned reporter. Studies are currently underway to image mice implanted with Gl261 mouse or U87 human glioma cells that have been transfected with the rdLRP and to image viral infection of rodent glioma models with an rdLRP expressing oncolytic virus.Acknowledgements
This work was supported by the National Institutes of Health grants R01-CA203873 and P41-EB015896.References
1. Gilad AA, McMahon MT, Walczak P, Winnard PT, Raman V, van Laarhoven HWM, Skoglund CM, Bulte JWM, van Zijl PCM. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol 2007;25:217–219. doi: 10.1038/nbt1277.
2. Minn I, Bar-Shir A, Yarlagadda K, Bulte JWM, Fisher PB, Wang H, Gilad AA, Pomper MG. Tumor-specific expression and detection of a CEST reporter gene. Magn Reson Med 2015:n/a–n/a. doi: 10.1002/mrm.25748.
3. Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers MLM, McMahon MT, Gilad AA, Fulci G. Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy. Radiology 2015;275:746–754. doi: 10.1148/radiol.14140251.
4. Yadav NN, Jones CK, Xu J, Bar-Shir A, Gilad AA, McMahon MT, van Zijl PCM. Detection of rapidly exchanging compounds using on-resonance frequency-labeled exchange (FLEX) transfer. Magn Reson Med 2012;68:1048–1055. doi: 10.1002/mrm.24420.
Figures