3047
GMP-grade nanoparticle imaging agent for 19F MR, photoacoustic, and fluorescence imaging1Tumor Immunology, Radboud Institute for Molecular Life Sciences, Nijmegen, Netherlands, 2Radiology and Nuclear Medicine, Radboud University Medical Center, Nijmegen, Netherlands, 3Medical Oncology, Radboud University Medical Center, Nijmegen, Netherlands
Synopsis
Cellular therapies hold great promise for the treatment of various diseases. Its success strongly depends on the imaging modality and cell tracking, which can be achieved by the addition of an imaging label to cells, for example in the form of nanoparticles. Here, we report on polymeric nanoparticles encapsulating perfluorocarbon and dye, which can be used for cell loading and can be detected with several imaging modalities. This will further give information about cell numbers and localization in vivo.
Introduction
Cellular therapies hold great promise for the treatment of various diseases, including cancer, diabetes mellitus, and cardiovascular diseases. The success of this form of therapy strongly depends on the imaging modality and cell tracking, which can be achieved by the addition of an imaging label to cells, for example in a form of nanoparticles (NPs). NPs encapsulating an imaging agent, after being loaded into the therapeutic cell, will give information about cells localization, number, and functionality.1,2,3 Recently we developed NPs with a 200 nm mean diameter, containing a liquid perflurocarbon entrapped in a polymer.4 These NPs are of special interest as they are clinically relevant, and their shape, size and surface chemistry can be modified. In this communication, we introduce the application of these NPs as contrast agent which can be used for in vivo multimodal imaging, using techniques such as fluorine magnetic resonance imaging, (19F MRI), photoacoustic imaging (PAI, sometimes also called optoacoustic), and fluorescence (FL) imaging.Materials and Methods
Poly(lactic-co-glycolic acid) NPs encapsulating perfluoro-15-crown-5 ether (PFCE) and Indocyanine green (ICG) were prepared using single emulsion solvent evaporation method and freeze-dried. To study the NPs application as contrast agents, NPs were resuspended in phosphate-buffered saline at various concentrations and placed in phantoms for imaging. The fluorine MR was performed on 11.7T MRI scanner (Biospec 117/16, 500 MHzBruker, Ettlingen, Germany). ZTE (zero echo time), TR=2 ms, flip angle 4°, matrix size=32 isometric voxels (voxel size=1.87 mm), number of averages=128, scan time=15 minutes, FOV=60x60x60 mm. PAI was performed on Vevo® 2100 LAZR Imaging scanner (VisualSonics®, Inc., Toronto, Canada) equipped with a LZ250 21 MHz transducer. Fluorescent imaging was done on IVIS LUMINA in vivo imaging system (excitation set at 645 nm, emission set for ICGreen). Immature monocyte-derived denritic cells (moDCs) were obtained from buffy coats of healthy individuals. On day 3 moDCs were harvested, counted and labeled with PLGA nanoparticles (resuspended in PBS shortly prior the labelling) at concentration 2 mg of nanoparticles/ 1x106 cells and incubated at 37°C for 72 hours. Wild-type C57Bl/6J female mice were obtained from Charles River Laboratory. 40 µl of nanoparticles in PBS at concentration 100 mg/ml or 1x106 and 0.1x106 cells (in 30 µl PBS) loaded with nanoparticles were injected into a mouse thigh muscle under ultrasound guidance. Immediately after the injection, mice were imaged using 19F MRI, PAI, and FL imaging.Results and Discussion
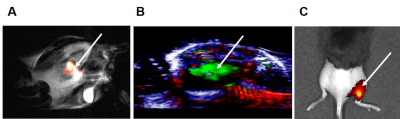
NPs were tested in a gel phantom and in vivo to measure their signal intensity and effectiveness as imaging agents in different imaging modalities. PLGA-PFCE-ICG NPs can be detected both in vitro and in vivo (Figure 1) with 19F MRI and PAI. As PFCE has a single resonance peak, due to its 20 equivalent 19F atoms, we could detect the 19F signal and overlay the 19F images with the corresponding regular 1H image (Figure 1A). In PAI (Figure 1B) the absorption peak from NPs was detected at 800 nm. Using the photoacoustic signal dependence upon the wavelength of the laser light used to illuminate the sample, we were able to unmix received signals into the individual absorption spectra of hemoglobin and NPs. This allowed clear detection of NPs within the tissue. Furthermore, these results were confirmed with the FL (Figure 1C) measurements, demonstrating the application of NPs also in this type of imaging. In vivo experiments showed that also cells loaded with NPs could be detected in tissue with these imaging modalities. Above results clearly show that PLGA-PFCE-ICG NPs can be used for cell labelling and imaged both in vitro and in vivo with several imaging modalities.Conclusion
In this study, we have demonstrated that PLGA-PFCE-ICG NPs are a potential candidate as an imaging agent for 19F MRI, PAI and FLI. PLGA-PFCE-ICG NPs are non-toxic and suitable for cells labeling, which makes them attractive for the use as in vivo cell tracers in cellular therapies. We are now producing them at GMP grade for human use. These NPs can be further adapted for targeting and drug delivery, by the addition of targeting ligands and encapsulation of hydrophobic compounds.Acknowledgements
This work was supported by the European Research Council (ERC) Starting Grant (CoNQUeST Grant no.336454) to MS. IJMdV received NWO-Vici 918.14.655.References
1: Srinivas M, Boehm-Sturm P, Figdor CG, de Vries IJ, Hoehn M. Labeling cells for in vivo tracking using (19)F MRI. Biomaterials. 2012 Sep 5.
2: Aarntzen EH, Srinivas M, et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3'-fluoro-3'-deoxy-thymidine ([18F] FLT) PET imaging. Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18396-9.
3: Srinivas M, Aarntzen EH, Bulte JW, Oyen WJ, Heerschap A, de Vries IJ, Figdor CG. Imaging of cellular therapies. Adv Drug Deliv Rev. 2010 Aug 30;62(11):1080-93. Review.
4: Srinivas, M. et al. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 31(27), 2010