3044
Unexpected accumulation of iron in liver of immune compromised mice: Implications for cell tracking experiments1Radiology, Michigan State University, East Lansing, MI, United States, 2Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, United States, 3Veterinary Diagnostic Laboratory, Michigan State University, East Lansing, MI, United States
Synopsis
An increased iron load was observed in immune-deficient mice which may mean that they are not suitable for iron oxide based cell tracking experiments. This was not seen in healthy controls fed a similar diet. It was resolved by feeding a low-iron diet.
Introduction
Cells transplanted into the liver and monitored using cellular MRI include islets, hepatocytes and macrophages.1-4 It is critical for iron oxide particle-based cell tracking to be able to differentiate cells from background tissue. Extremely immune-deficient mice, such as NOD/SCID IL-2γ knockout mice (NOG), are required as recipients of cells of human or porcine origin. However, we noticed a phenotype of extreme liver iron accumulation in our colony of NOG mice that would completely prevent iron oxide particle cell tracking. We quantified the iron accumulation in immune compromised and wild-type mice using T2*-weighted imaging and T2* maps and tested the effect of a low-iron diet on the excessive liver iron.Methods
Mice were scanned in a 7T Bruker Biospec 70/30 USR using a birdcage transmit coil and four channel array receive coil, using respiratory gating. T1-weighted FLASH images were acquired with TR/TE=300/6 ms, FA 30°, 10 averages, 100x100x300 µm resolution. T2* maps were acquired on the same slices using a multi-gradient echo sequence with echo times from 1.63 ms and echo spacing every 1.63 ms, and 8-12 positive echoes were acquired.
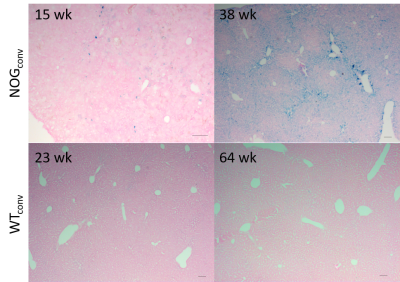
Three populations of mice on different diets were studied. 1) A cross-sectional study of a colony of NOG mice ages 9-31 wk that were fed one of two conventional commercial diets used in our animal facilities (280 or 290 mg Fe/kg) (NOGconv) (n= 27). 2) A cross-sectional study of a colony of C57/bl6-GFP mice (11-64 wk) that were fed a conventional diet (200 mg Fe/kg) (WTconv) (n= 10). 3) A prospective study of a group of NOG mice fed a low-iron diet (6 mg Fe/kg) from age 6 wks and scanned every 2 weeks (NOGlowFe) (n=8 thus far). Livers were collected for cryosectioning and staining with Perls’ Prussian blue.
Results
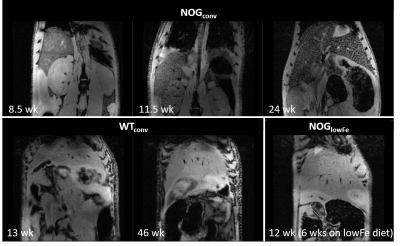
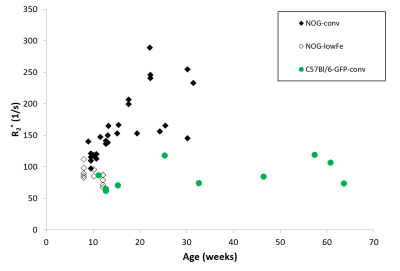
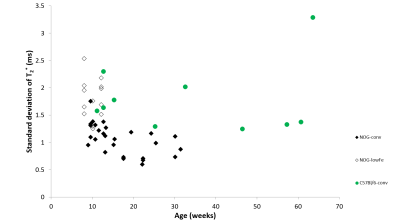
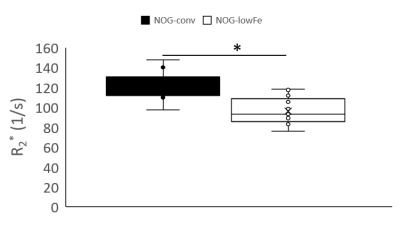
NOG mice aged as early as 13 weeks had an excess of iron in their livers, visible as mottling in T2* weighted images. The effect worsened as the mice aged. There was no difference in liver appearance with age in the wild-type mice, and there was no accumulation of iron in the livers of NOG mice on the low-iron diet (Figure 1). There was an increase in R2* with age in the NOGconv mice, with a correlation coefficient of 0.5. There was only a mild correlation between age and R2* in the WT mice fed the conventional diet (r2=0.2). We have preliminary results for the change in R2* of the NOGlowFe group so no correlation was calculated (Figure 2). We measured the texture of the T2* maps by calculating the standard deviation of the T2* (Figure 3). The T2* variability decreased with age in the NOGconv mice; coupled with the overall increase in R2*, this suggests that the size of the hypointensities increases with age. To examine the effect of the low iron diet, we compared the R2* of young (aged 8-12 weeks) NOGconv and NOGlowFe mice (Figure 4). The liver R2* of the mice on the low iron diet was significantly lower than that of the mice on the conventional diet. Perls’ staining confirmed that there was abundant iron in the livers of the NOGconv mice that was not seen in the WTconv mice. Mice fed the low iron diet have so far not shown any adverse effects such as weight loss or poor body condition.Discussion
Liver R2* dramatically increased in NOGconv mice as they aged, and was visible in gradient echo images as early as 12 weeks old. R2* is directly related to hepatic iron content5. This would make it impossible to see transplanted iron oxide labeled cells by MRI. This effect was not seen in C57Bl/6-GFP mice fed a conventional diet. Our early results suggest that the phenotype can be rescued by feeding the NOG mice a low-iron diet. Future investigations could examine the use of a medium-iron diet so that any adverse effects from the diet, such as anemia, are minimized. The mechanism for this high hepatic iron is still unknown. The implications for long-term health of the mice are unknown; excessive liver storage of iron is related to fibrosis and inflammation6.Acknowledgements
Funding from NIH R01 DK107697References
1. Evgenov, N. V. et al. In Vivo Imaging of Immune Rejection in Transplanted Pancreatic Islets. Diabetes 55, 2419–2428 (2006).
2. Luciani, A. et al. In vivo imaging of transplanted hepatocytes with a 1.5-T clinical MRI system--initial experience in mice. Eur Radiol 18, 59–69 (2008).
3. Sharkey, J. et al. Functionalized superparamagnetic iron oxide nanoparticles provide highly efficient iron-labeling in macrophages for magnetic resonance–based detection in vivo. Cytotherapy 19, 555 (2017).
4. Ju, S. et al. In vivo differentiation of magnetically labeled mesenchymal stem cells into hepatocytes for cell therapy to repair damaged liver. Invest Radiol 45, 625–633 (2010).
5. Wood, J. C. et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106, 1460–1465 (2005).
6. Sirlin, C. B. & Reeder, S. B. Magnetic Resonance Imaging Quantification of Liver Iron. Magnetic resonance imaging clinics of North America 18, 359 (2010).
Figures