3041
Camelid single-domain antibodies bioconjugate for the magnetic resonance imaging of Alzheimer’s disease.1Molecular Imaging Research Center (MIRCen), Commissariat à l'énergie atomique et aux énergies alternatives (CEA), Fontenay aux Roses, France, 2Unité de Chimie des Biomolécules, Institut Pasteur, Paris, France, 3Institut du cerveau et de la moelle épinière (ICM), Paris, France, 4Plateforme d’Ingénierie des Anticorps, Institut Pasteur, Paris, France
Synopsis
Detection of intracerebral targets with imaging probes is challenging due to the non-permissive nature of blood-brain barrier (BBB). Camelid single-domain antibody-fragments (VHH) are small and stable antibodies able to potentially cross the BBB. Here, we selected VHH specifically targeting amyloid-beta deposits, one of the main lesions of Alzheimer’s disease and labeled them with the contrastophore gadolinium. These innovative contrast agents allowed MRI detection of amyloid deposits in postmortem brain tissues of a mouse model of amyloidosis. The ability to produce VHH conjugates that cross the BBB opens the way for future development of tailored imaging probes targeting intracerebral antigens.
Introduction
Amyloid plaques are one of the main pathological hallmarks of Alzheimer’s disease (AD). Detection of these lesions by neuroimaging is a very promising biomarker of this pathology. Prior studies attempted to design specific contrast agents for high-resolution MR imaging of individual amyloid plaques1-3. One class of promising antibodies for in vivo imaging is camelid single-domain antibody-fragments (VHH). We previously demonstrated that some VHH with a basic isoelectric point can readily transmigrate across the BBB4,5. In this study, we selected a basic VHH specifically targeting amyloid-β (Aβ) peptide and we designed several gadolinium (Gd)-VHH conjugate MRI probes. Contrast agents are characterized by their relaxometric parameters r1 and r26. A relaxometric study allowed us to select the conjugate with the higher relaxometric properties. Then, we demonstrated that these conjugates cross the BBB and allow postmortem detection of their target after systemic injection in living mice. Finally, we investigated their efficiency to detect amyloid deposits in vitro by MRI.Methods
After immunization with a fibrillar synthetic Aβ42 peptide, VHH that specifically recognize Aβ were selected by phage-display. Soluble VHH were then expressed in E. coli with a 6-Histidine tag to allow its purification and immunodetection. Finally, VHH were conjugate with maleimide-(DOTA/Gd)3. First, the capacity of VHH to detect their target was assessed by immunohistochemistry after incubation of the brain sections from PS2APP mouse and from patients with AD with VHH and their detection by an anti-VHH antibody.To evaluate the capacity of VHH to cross the BBB, a 50-mg/kg dose (or PBS) was injected intravenously into two PS2APP mice. Four hours after injection, mice were sacrificed, and paraffin brain sections were prepared.Then, longitudinal and transversal relaxivities (r1 and r2) of the different (Gd)-VHH conjugates were assessed with a 11.7T spectrometer. T1 calculation was based on a T1 fast spin echo sequence (TR=300, 500, 800, 1500, 3000, 5000msec, TE=7 msec, RARE factor=2, FOV=15x15mm2, Mtx=256x256 and slice thickness=1mm). T2 calculation was based on a T2 multi slice multi echo sequence (TR=2500msec, thirty TE=7.5 to 223msec, bandwidth=96kHz, FOV=15x15mm2, Mtx=256x256, slice thickness=1mm). Dotarem® (Guerbet, France) was used as reference. Finally, brains from PS2APP were incubated with (Gd)-VHH conjugates (n=2) or PBS (n=2). Brains of wild-type mice were also incubated with (Gd)-VHH conjugates as control. MR images of these brains were recorded at a resolution of 25×25×25µm3 using a 3D-FLASH sequence (TR=40 msec, TE=15 msec, Nex=4, bandwidth=37.5 kHz, FOV=12.8x12.8x6.4 mm3, Mtx=512x512x256) . Following MRI, brain tissues were processed by histology.
Results
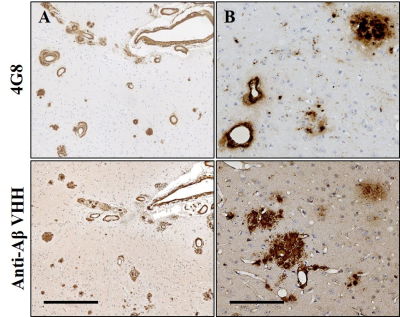
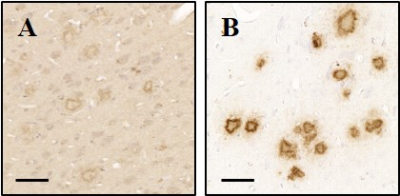
To assess the binding properties of VHH, immunohistochemistry was performed on brain tissue sections from PS2APP and from patients with AD. VHH showed excellent sensitivity and selectivity in detecting amyloid plaques and cerebral amyloid angiopathy both on mouse and human sections (Fig. 1). We assessed the capacity of (Gd)-VHH conjugates to cross the BBB in vivo after intravenous injection in PS2APP mice. In animals injected with VHH, immunostaining using anti-VHH antibody showed extensive plaque labeling throughout the brain (Fig. 2). No staining was found in mice injected with PBS.
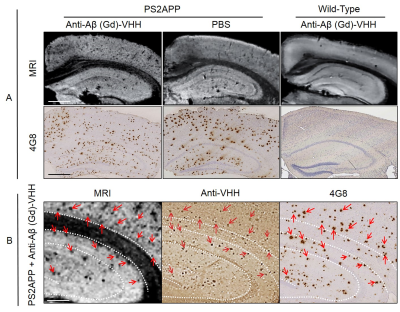
Relaxometric study allowed us to determine the r1 and r2 relaxivities of each (Gd)-VHH conjugate. The r1 and r2 relaxivities of conjugates were 5, 4.3, 5.4 and 4.25 and 25, 19, 17 and 17 respectively, wich is 1.9 to 2.5 and 4.25 to 6.25 higher than those of DOTAREM. Finally, we investigated the efficiency of the (Gd)-VHH conjugates to detect amyloid deposits by MRI in vitro. In brains incubated with (Gd)-VHH conjugates, numerous hypointense spots were easily observed throughout the brain. No hypointense spot were detected in the brain of wild-type mice after incubation with (Gd)-VHH conjugates. The density of hypointense spots detected on MR images was increased sevenfold in PS2APP hemispheres incubated with VHH compared to PS2APP hemispheres incubated in PBS. Registration between MR images and histological sections showed that hypointense spots detected by MRI co-localize with amyloid plaques. Taken together, these results demonstrate a strong increase of amyloid plaque detection in MR images of PS2APP brains following incubation with (Gd)-VHH conjugate in vitro (Fig 3).
Discussion and conclusion
We described a novel anti-Aβ (Gd)-VHH that binds the Aβ peptide with high specificity and sensitivity and labels amyloid deposits on brain section of a mouse model of amyloidosis or AD patients. Furthermore, we demonstrated its ability to cross the BBB and to label amyloid deposits after intravenous injection in mice. Finally, we showed that our (Gd)-VHH conjugates display high relaxivities allowing the detection of amyloid deposits in vitro by MRI. Further development for in vivo imaging applications still relies on several optimizations but the ability to produce VHH conjugates that cross the BBB opens the way for future development of imaging probes targeting intracerebral antigens.
Acknowledgements
No acknowledgement found.References
1. Poduslo J.F., et al. Molecular targeting of Alzheimer's amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiol Dis. 2002 Nov; 11(2):315-29.
2. Sigurdsson E.M., et al. A non-toxic ligand for voxel-based MRI analysis of plaques in AD transgenic mice. Neurobiol Aging. 2008 Jun;29(6):836-47.
3. Wadghiri Y.Z., et al. Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. PloS One. 2013;8(2):e57097.
4. Li T., et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J Control Release. 2016 Dec 10;243:1-10.
5. Li T., et al. Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: application to brain imaging. FASEB J. 2012 Oct;26(10):3969-79.
6. Caravan P., Ellison J.J., McMurry T.J., Lauffer R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev 1999; 99:2293-352.
Figures