3039
A Small Molecule NIR-19F MR Contrast Agent of Aza-BODIPY for Bimodal In Vivo Imaging1State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences-Wuhan National Laboratory for Optoelectronics, Wuhan, China, 2Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Accurate
and early diagnosis of diseases is most import for medical imaging. MRI is one
of the most promising techniques for the non-invasive visualization. Compared
to 1H MRI, 19F MRI provides high-contrast images without
endogenous background signals, but low sensitivity. To address the limitation,
our strategy is to combination of 19F MRI and a more sensitive NIR
fluorescence imaging technique to develop a bimodal contrast agent BDPF. Both ex
vivo and in vivo experimental results indicated BDPF had excellent optical and 19F
MRI properties. Thus, the NIR-19F
MR bimodal imaging may provide a new way to detect tumor.
INTRODUCTION
Early detection requires imaging techniques with higher sensitivity, while maintaining specificity for disease states. Multimodality imaging based on complementary detection principles has broad clinical applications and promises to improve diagnostic accuracy.1 Synergistically combining multiple imaging modalities can enhance the overall diagnostic information. MRI is a noninvasive imaging modality that provides excellent contrast for soft tissues.2 Recently, 19F has received significant attention for MR imaging agents owing to the low levels of endogenous fluorine in the body and the direct relationship between 19F signal and agent concentration.3 A significant limitation of MRI is the low sensitivity. One strategy to address this shortcoming involves multiplexing an MR contrast agent with a more sensitive imaging modality such as optical imaging. As a NIR dye, aza-BODIPY has attracted considerable attention in recent years for the detection of neutral molecules, metal cations, anions, and pH values. Herein, we proposed a small molecule NIR-19F MR contrast agent of aza-BODIPY which displayed excellent optical and 19F NMR properties. Additionally, we demonstrated that the BDPF could be well internalized by human lung cancer cells A549 cells and exhibited a negligible toxicity. This facilitated our first in vivo detection of this bimodal imaging agent.
RESULTS AND DISCUSSION
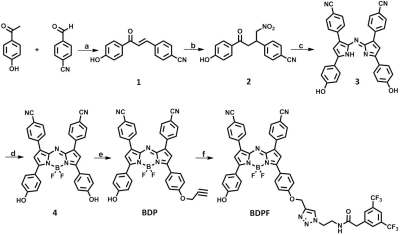
For the achievement of NIR fluorescence and 19F MR bimodal imaging, we first synthesized an aza-BODIPY-based contrast agent BDPF through the strategy illustrated in Scheme 1.
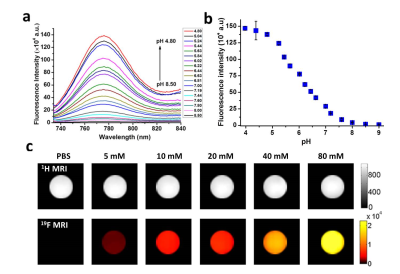
The optical properties of BDPF have been investigated using absorption and emission spectra. BDPF displayed excellent photophysical properties and good photostability. As is shown in the spectra over different pH values in Figure 1a, b, BDPF is a pH-responsive fluorescence contrast agent. 19F MR imaging on phantoms showed the intensity successively increased as BDPF concentration increase (Figure 1c). No 19F MRI signal was detected in the control solution, suggesting the signal was only come from BDPF.
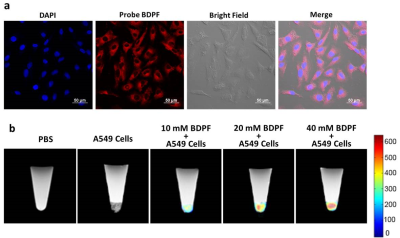
Next, the in vitro NIR-19F MR bimodal imaging capability of BDPF was assessed in cultured cell lines. The cellular uptake and intercellular localization of the contrast agent were observed by confocal laser scanning microscopy (CLSM). A549 cells have an efficient cellular uptake of BDPF and mainly internalized into cytoplasm (Figure 2a). In vitro 19F MRI experiments were performed at 9.4 T (Figure 2b). No 19F MRI signal was observed in control groups, whereas for other samples, the signals were clearly observed and successively increased as BDPF concentration increase. These results reconfirm that BDPF could be well internalized by A549 cells and have good biocompatibility.
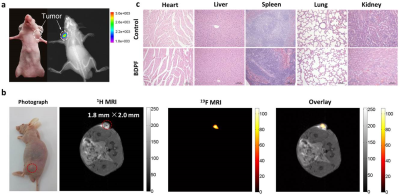
Finally, to demonstrate that BDPF was sufficiently sensitive for in vivo NIR-19F MR bimodal imaging, biomodal imaging was performed using an A549 tumor-bearing mouse Both NIR fluorescence and 19F MRI images revealed a clear signal in the tumor region owing to the low background in vivo (Figure 3a, b). Histological analysis revealed that BDPF had negligible toxicity and side effects for in vivo bimodal imaging (Figure 3c).
CONCLUSION
In summary, we have developed a bimodal imaging agent, BDPF, for in vivo NIR-19F MR imaging. This agent possessed excellent photophysical properties, a pH sensitive fluorescence spectra and high 19F MRI sensitivity. The cellular assays revealed the contrast agent could be efficiently internalized by A549 cells and displayed good biocompatibility. In addition, we have demonstrated for the first time that BDPF could detected in vivo in a small tumor (smaller than 2 mm), combining the sensitivity of NIR fluorescence and the high resolution of 19F MRI. Thus, the NIR-19F MR bimodal probe BDPF may provide a new way for detecting tumors by small molecular imaging agents.Acknowledgements
No acknowledgement found.References
[1] a) M. S. Albert et al. Nature 1994, 370, 199-201; b) X. Zhou et al, NMR Biomed. 2011, 24, 170-175
[2] a) K. Bartik et al, J.Am.Chem.Soc. 1998, 120, 784-791; b) Y. F. Wang et al, Chem Commun. 2015, 51, 8982-8985; c) M. G. Shapiro et al, Nat. Chem. 2014, 6, 629 – 634; d) Y. Wang et al, Angew. Chem. Int. Ed. 2016, 55, 8984–8987; e)T. K. Stevens et al, J. Am. Chem. Soc. 2013, 135, 9576–9579; f) S. Klippel et al, Nano Lett. 2014, 14, 5721–5726.
[3] a) X. Zhou et al, Anal. Chem. 2016, 88, 5835-5840; b) X. Zhou et al, Anal. Chem. 2017, 89, 2288–2295; c) X. Zhou et al, Chem. Eur. J. 2016, 22, 3967- 3970
Figures