3037
A Hyperpolarized 129Xe “OFF-ON” MRI Biosensor Triggered by Diamine Oxidase1State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, 430071, Wuhan China-Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology (HUST), 430074, Wuhan, China, 2State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, 430071, Wuhan, China
Synopsis
Benefiting from ultra-high sensitivity of Hyper-CEST method, 129Xe biosensors possess an obvious advantage in sensitivity over other MRI sensors. However, due to its indirect detection mode the Hyper-CEST spectra resolution is relatively low limiting chemical shift to be an effective indicator in traditional NMR. In order to solve this problem, a 129Xe biosensor based on a new “turn-on” strategy is designed, which exhibits high detection specificity for an enzyme diamine oxidase (DAO). This 129Xe biosensor possesses very high detection sensitivity, and can be tested in Small intestinal villus epithelial cells. Using this strategy, lots of disease-related enzyme can be detected.
Introduction
Benefiting from being noninvasive imaging techniques and excellent tissue penetration, Magnetic resonance imaging (MRI) has been a well-established clinical imaging method. However, valuable insight for disease diagnosis is blocked. Hyperpolarized 129Xe NMR and MRI have appeared and been studied in an increasingly wide utilization. Meanwhile, chemical shift have been used for diverse applications such as detection of other analyte and marker imaging. Nevertheless, low spectra resolution limits the chemical shift to be an effective indicator in traditional NMR.1-3 In order to solve this problem, a new biosensor relying on CB6 supramolecular host-guest interactions was described.Methods
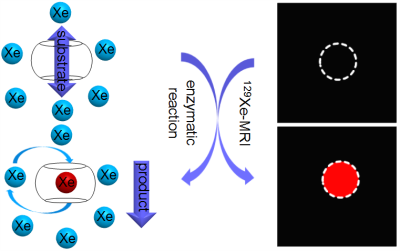
As depicted in Figure 1, in order to create 129Xe@cage “OFF-ON” MRI biosensor for enzyme detection, the binding constant of substrate and cage should be much larger than that of product and cage. When the substrate binds with cage, the 129Xe atom can’t get into the cavity of cage to give the MRI signal of caged 129Xe. After the enzymatic reaction, the affinity of product and cage is not so strong, which makes the 129Xe atoms exchange effectively in and out the cage cavity. As a result, the MRI signal of caged 129Xe can be detected. Cucurbituril[6] (CB6) was chosen as 129Xe-binding cage. Diamine oxidase (DAO) was chosen as the target and putrescine dihydrochloride (PUT) as substrate of enzymatic reaction.Results and Discussion
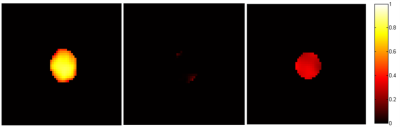
Figure 2 is the main result of our experiment in buffer. Hyper-CEST 129Xe MRI images (a) CB6, (b) CB6+PUT and (c) CB6+PUT+DAO, were obtained. The difference between a and b shows that 129Xe@CB6 MRI signals can be completely suppressed by PUT. Moreover, the difference between b and c illustrates that DAO can be specifically detected and localized by Hyper-CEST 129Xe MRI.
The property of biosensor was also investigated in Small intestinal villus epithelial cells by Hyper-CEST 129Xe MRI. As showed in Figure 3, the difference between a and b suggests that 129Xe@CB6 MRI signals can be completely suppressed by PUT, while hydroxylamine hydrochloride (Hyd) inhibits the activity of DAO. Moreover, the difference between b and c illustrates that 129Xe@CB6 MRI signals can be lighted by DAO in cell.
Conclusion
The results represented here prove that 129Xe@CB6 MRI signals can be entirely inhibited by the substrate of enzyme. When a specific enzymatic reaction occurs the CB6 is released to produce a 129Xe@CB6 signal. This 129Xe MRI sensor trigger should be endogenous and easily discovered in laboratory. Consequently, it suggests that CB6 could be an excellent biosensor in 129Xe MRI detection. A lot of enzyme can be detected by this “OFF-ON” 129Xe@CB6 Hyper-CEST MRI mode.Acknowledgements
This work was funded by the National Natural Science Foundation of China (81227902, 81625011), National Program for Support of Eminent Professionals (National Program for Support of Top‐notch Young Professionals)References
[1] Qianni Guo, et al. Chemistry - A European Journal, 2016; 22: 3967-3970.
[2] Ji Zhang, et al. Talanta, 2014; 122: 101-105.
[3] Matthias Schnurr, et al. Angewandte Chemie International Edition, 2015; 10: 13444-13447.
Figures