3035
Prospects of 31P Contrast Media for 31P-MRS1Chemistry Research Laboratory, University of Oxford, Oxford, United Kingdom, 2BHF Experimental MR Unit (BMRU), University of Oxford, Oxford, United Kingdom, 3Physical and Theoretical Chemistry Laboratory, University of Oxford, Oxford, United Kingdom, 4Radiobiology Research Institute, University of Oxford, Oxford, United Kingdom, 5Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom
Synopsis
31P-MRS can be used to determine the relative ratios of phosphate species in vivo to aid clinical diagnosis, but is limited by poor SNR and long acquisition times associated with the 31P nucleus. This work investigates the potential of 31P T1 contrast agents based on Gd.DO3A derivatives by using 31P-MRS. These compounds demonstrate significant relaxation enhancement of 31P R1 for ATP, PCr and Pi, therefore showing excellent potential as 31P contrast agents. Cell studies indicate the Gd.DO3A derivatives investigated do not come into contact with intracellular phosphate metabolites, which limits these initial complexes to use as extracellular contrast agents.
Introduction
31P magnetic resonance has yet to realise its clinical potential principally due to the relatively low sensitivity of the 31P nucleus. While 31P-MRS can uniquely provide vital information regarding in vivo phosphorus metabolism, the long acquisition times required are incompatible with observing dynamic changes in phosphorus speciation (e.g. monitoring ATP/PCr ratios in real time).1 This study reports how heptadentate gadolinium (Gd) complexes can be used to enhance 31P relaxivity, reducing T1 values by more than an order of magnitude per millimolar Gd. This enables shorter repetition times markedly improving the signal to noise ratio (SNR) per unit time.Methods
A series of Gd complexes derived from DO3A were designed and synthesised to be 31P T1 contrast agents (Figure 1) on the basis of their affinity for phosphate species and the likelihood of fast phosphate exchange on the timescale of the NMR experiment.2 We studied the binding of phosphate and measured the resulting 31P T1 relaxation rates of ATP, PCr and Pi as a function of Gd concentration. For comparison, commercial contrast agents were also tested (Dotarem® (Gd.DOTA), Omniscan® (Gd.DTPA-BMA) and Magnevist® (Gd.DTPA)). The uptake of the DO3A derived contrast agents into cells was studied using diffusion measurements combined with T1 measurements to assess the degree of uptake, and these results correlated with fluorescent microscopy experiments on luminescent analogues containing europium in lieu of gadolinium.Results
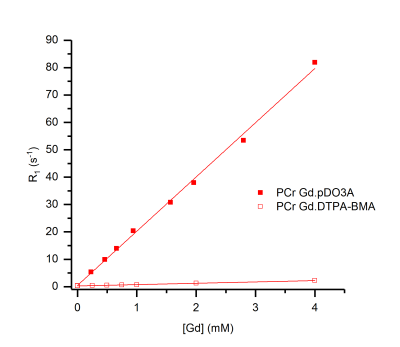
Dramatic reductions in 31P T1 values were observed for the Gd.DO3A derived complexes for all phosphate species studied. A linear correlation was observed between Gd-complex concentration and R1 for these complexes. Dotarem exhibited smaller, but linear, enhancements to R1 under the same conditions. However, Magnevist and Omniscan exhibited pronounced non-linearity of R1 enhancement for ATP with increasing Gd-complex concentration (Figure 2). Cellular uptake is observed for some DO3A complexes. Fluorescence microscopy of europium analogues reveals that internalisation occurs into endosomes, consistent with the pinocytosis pathway as observed previously.3 MR measurements and diffusion studies reveal that there is no significant change to 31P T1 relaxation in cells following exposure to contrast agent.Discussion
These results are consistent with binding of phosphate to Gd.DO3A derivatives in fast exchange on the NMR timescale, resulting in a net reduction of the observed T1 of the phosphate in solution in a manner analogous to 1H T1 contrast enhancement in MRI. Dotarem shows a weaker 31P T1 effect than Gd.DO3A derivatives, that is almost certainly attributable to outer sphere phosphate interactions (and which is further limited by electrostatic repulsion between the anionic Dotarem complex and phosphate anions). The non-linear plots of R1 versus Gd concentration observed for Magnevist and Omniscan are consistent with competitive binding of ATP to Gd; kinetic instability of these contrast agents can be inferred from this observation.4 The cellular internalisation of Gd.DO3A derivatives into endosomes indicates that cell-uptake does not lead to contrast enhancements for intracellular phosphate species, which reside in the cytoplasm and mitochondria. However, such contrast agents could therefore be used to distinguish intra- and extra-cellular phosphate species.Conclusion
These results show that Gd.DO3A systems have clear potential as 31P T1 contrast agents. For the agents described above, there is potential to provide information regarding the localisation of 31P metabolites in vivo, observed by 31P-MRS. Clearly, it is desirable to define cell uptake pathways that allow intracellular phosphate to be measured effectively. This will require a new generation of gadolinium based probes that incorporate structural motifs to target/enhance cellular internalisation. Competitive binding by ATP to Gd in some clinical contrast agents suggests the possibility that 31P R1 measurements may be used to assess contrast agent stability for application in vivo.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC), the Medical Research Council (MRC) [grant number EP/L016052/1] and the British Heart Foundation (FS/11/50/29038).References
1. Bottomley PA, Hardy CJ, Roemer PB, Weiss RG. Problems and expediencies in human 31P spectroscopy. The definition of localized volumes, dealing with saturation and the technique-dependence of quantification. NMR Biomed. 1989;2:284–289.
2. Bruce JI, Dickins RS, Govenlock LJ, et al. The selectivity of reversible oxy-anion binding in aqueous solution at a chiral europium and terbium center: Signaling of carbonate chelation by changes in the form and circular polarization of luminescence emission. J. Am. Chem. Soc. 2000;122:9674–9684.
3. New EJ, Congreve A, Parker D. Definition of the uptake mechanism and sub-cellular localisation profile of emissive lanthanide complexes as cellular optical probes. Chem. Sci. 2010;1:111-118.
4. Elst LV, van Haverbeke Y, Goudemant JF and Muller RN. Stability assessment of gadolinium complexes by P-31 and H-1 relaxometry. Magn. Reson. Med. 1994; 31: 437–444.
Figures
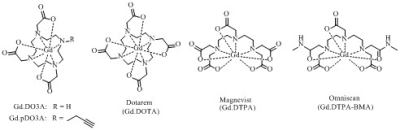
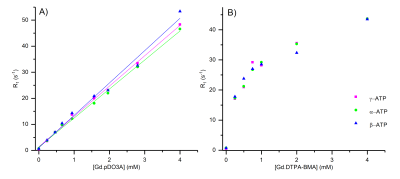
Figure 2: R1 of α-, β-, γ-ATP (9.9 mM) with increasing Gd concentration at pH 7.2, 9.4 T, where Gd is A) Gd.pDO3A and B) Gd.DTPA-BMA (Omniscan). The linear fits of α-, β-, γ-ATP with Gd.pDO3A give relaxivity (r1) values ± standard error of 11.14 ± 0.22, 12.42 ± 0.53 and 11.65 ± 0.23 respectively.