3034
Benzene-Appended Cucurbit[6]uril as a Potential Biosensor Scaffold for Hyperpolarized 129Xe MRI Molecular Contrast Agents1Chemistry, Lakehead University, Thunder Bay, ON, Canada, 2Chemistry, University of Rhode Island, Kingston, RI, United States, 3Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 4Biology, Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
We have recently advanced the field of hyperpolarized (HP) 129Xe magnetic resonance imaging (MRI) with the in vivo detection of cucurbit[6]uril (CB6), a highly sensitive MR contrast agent. CB6 is biochemically inactive, which makes its natural bio-distribution non-specific; thus, it cannot be precisely localized within a living mammalian body using HP 129Xe MRI. We have previously identified cyclodextrin-based pseudorotaxanes as conjugatable scaffolds for xenon biosensors; in this work, we introduce a second class of conjugatable scaffolds, with the hyperCEST detection of benzene-appended CB6, a potential precursor to a wide variety of targeted molecular imaging probes.
Introduction
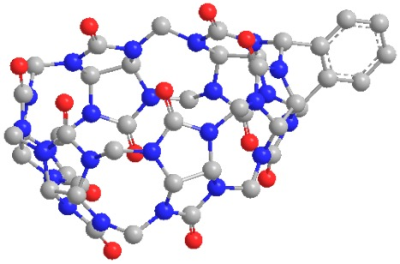
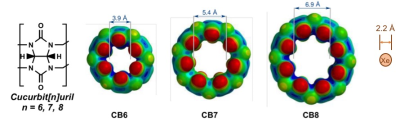
Hyperpolarized (HP) 129Xe magnetic resonance imaging (MRI) has the potential to become a clinical molecular imaging modality with comparable sensitivity to positron emission tomography (PET), through the development of targeted molecular imaging probes, known as xenon biosensors (XB).1-3 Compared to PET, however, MRI is capable of achieving submillimeter spatial resolution and it operates without exposure to potentially harmful ionizing radiation. Cucurbit[6]uril (CB6) is a highly sensitive MR imaging contrast agent, which we have demonstrated in a living mammalian model to validate proof of principle for this concept.4 However, the natural bio-distribution of CB6 in vivo is non-specific; thus, it cannot be precisely localized with HP 129Xe MRI. To advance this technology into a molecular imaging technique, the supramolecular host must be capable of interacting with specific biological media, often achieved via conjugation to an antibody or affinity tag. The development of a modular scaffold for XBs has been hindered due to various implications associated with synthesis, toxicity, and water-solubility. We have recently identified cyclodextrin-based pseudorotaxanes as easily conjugatable scaffolds for XBs5; however, the structure of these molecules involves an alkyl chain threaded through the center of the supramolecular cavity, which may influence xenon binding. In this work, we introduce a second class of non-toxic and water-soluble XB scaffolds, which are based on a monofunctional CB6 derivative6 (Figure 1). In this approach, benzene is directly coordinated to the periphery of the supramolecular cage, which preserves the integrity of the inner cavity and thereby alleviates potential influences on xenon binding.
Experimental Method
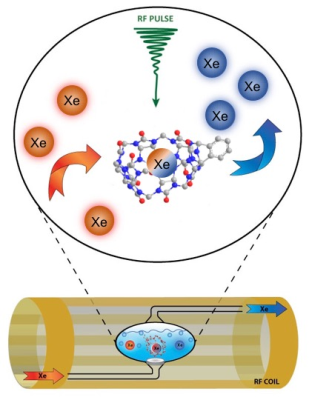
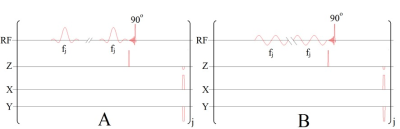
This investigation was conducted using a Philips Achieva whole-body clinical MRI scanner (3.0 T), which bodes well for eventual translation of our technique into clinical studies. Prior to the acquisition of experimental data, naturally abundant 129Xe gas was polarized to 47-50% using a Xemed polarizer (Xemed, Durham, NH, USA). Spectral data were acquired in vitro using a glass frit phantom, which was placed inside of a custom-made quadrature RF coil tuned to the resonance frequency of 129Xe (35.33 MHz) at 3.0 T and positioned in the center of the bore. A 2.5 mL sample of benzene-appended CB6 (10 mM), dissolved in DI water, was drawn into the phantom vessel and HP 129Xe gas was continuously bubbled into solution while hyperCEST depletion spectra were simultaneously acquired (Figure 2). Immediately prior to the acquisition of spectral data, a saturation pre-pulse sequence consisting of either 3-lobed sinc-pulses (Figure 3A) or continuous sinusoidal pulses (Figure 3B) was applied. Raw data were initially processed in MATLAB (MathWorks, Natick, MA, USA) and further filtered in OriginPro 2016 (OriginLab Corp, Northampton, MA, USA). Lorentzian fittings were applied to the filtered data to illustrate a smoothed depletion spectrum.Results & Discussion
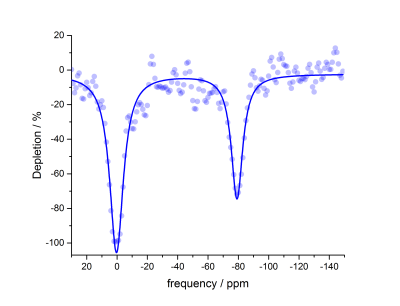
HyperCEST depletion spectra were acquired using various pulse sequence parameters, including pulse length duration, offset frequency step, repetition time (TR) and flip angle (FA). Maximum depletion for benzene-appended CB6 was observed to be 71.5 % at -80 ppm from the dissolved-phase 129Xe signal (0 ppm) using a 16- 30 ms sinusoidal pulse sequence with TR and FA equal to 4000 ms and 1530°, respectively (Figure 4). CB6 has been repeatedly demonstrated as an effective HP 129Xe contrast agent4,7, due to the compatibility of its cage size with the diameter of HP 129Xe atoms (Figure 5). As compared to other supramolecular scaffolds in the cucurbit[n]uril family, CB6 has been shown to most effectively bind HP 129Xe atoms (ka = 200 M-1)8. The nature of the coordination of benzene to the supramolecular scaffold of CB6 is advantageous because it does not interfere with the size of the cage cavity; thus, the binding of xenon with benzene-appended CB6 is theoretically unaffected. This unique property of the benzene-appended CB6 scaffold offers a creative approach to coordinate antibodies or affinity tags, which provide the ability of these molecules to specifically target disease receptors. Ultimately, the hyperCEST detection of benzene-appended CB6 marks a promising advancement in HP 129Xe biosensor technology, which is imperative for eventual translation of this technique into a molecular imaging modality.Conclusions
We disclose a potentially conjugatable scaffold for HP 129Xe molecular imaging agents, which is based on a benzene-appended monofunctional CB6 derivative. To our knowledge, this is the first demonstration of benzene-appended CB6 as a viable candidate for further research as a conjugatable scaffold for HP 129Xe molecular imaging probes. Given the efficacy of benzene-appended CB6 as a HP 129Xe contrast agent in vitro, we anticipate the in vivo demonstration of a targeted molecular imaging probe based on a similar coordination scheme to detect areas of pathology within living disease models.Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery grant and a Northern Ontario Academic Medicine Association (NOAMA) grant. BRJP is funded through an NSERC of Canada Undergraduate Student Research Award (USRA). FTH is supported by Canadian Institutes for Health Research (CIHR) and BrightFocus fellowships. The authors would like to thank Lakehead University (LU) and the Thunder Bay Regional Health Research Institute (TBRHRI) for partial support of this work and access to their facilities.References
- Schröder, L. in Albert, M.S. and Hane, F.T. eds. (2016) “Hyperpolarized and Inert Gas MRI: From Technology to Application in Research and Medicine.” Acad. Press. Ed. 1, pp. 263-277.
- Schröder, L., Lowery, T.J., Hilty, C., Wemmer, D.E., and Pines, A. (2006) Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science, 314(5798), pp. 446-449.
- Rose, H.M., Witte, C., Rossella, F., Klippel, S., Freund, C., and Schröder, L. (2014) Development of an Antibody-based, Modular Biosensor for 129Xe NMR Molecular Imaging of Cells at Nanomolar Concentrations. Proc. Natl. Acad. Sci. 111(32), pp. 11697-11702.
- Hane, F.T., Li, T., Smylie, P., Pellizzari, R.M., Plata, J.A., DeBoef, B. and Albert, M.S. (2017) In vivo Detection of Cucurbit[6]uril, a Hyperpolarized Xenon Contrast Agent for a Xenon Magnetic Resonance Imaging Biosensor. Sci. Rep., 7.
- Prete, B.R.J., Chahal, S., Fernando, A., Li, T., Hane, F.T., DeBoef, B., and Albert, M.S. (2017) Cyclodextrin-based Pseudorotaxanes as Conjugatable Molecular Imaging Biosensors for Hyperpolarized 129Xe MRI. Proc. Intl. Soc. Mag. Reson. Med. 25; 3051.
- Lucas, D., Minami, T., Iannuzzi, G., Cao, L., Wittenberg, J.B., Anzenbacher Jr, P. and Isaacs, L. (2011) Templated Synthesis of Glycoluril Hexamer and Monofunctionalized Cucurbit[6]uril Derivatives. J. Am. Chem. Soc., 133(44), pp. 17966-17976.
- Hane, F.T., Smylie, P.S., Li, T., Ruberto, J., Dowhos, K., Ball, I., Tomanek, B., DeBoef, B. and Albert, M.S. (2016). HyperCEST Detection of Cucurbit[6]uril in Whole Blood Using an Ultrashort Saturation Pre-pulse Train. Contrast Med. & Mol. Imaging, 11(4), pp. 285-290.
- El Haouaj, M., Luhmer, M., Ko, Y.H., Kim, K., Bartik, K. (2001). NMR Study of the Reversible Complexation of Xenon by Cucurbituril. J. Chem. Soc. Perkin Trans. 2(5), pp. 804-807.
Figures