3033
Smart thermosensitive liposomes for effective solid tumor therapy with MRI tracking at 21.1 T1The National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL, United States, 3University of Texas MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Here we show the ability of using Gadolinium (Gd) labeled thermosensitive liposomal nanoparticle (TSLnp) as a delivery system for anticancer drug, gemcitabine (Gem) to human pancreatic tumors. Pancreatic cancer (PCa) due to its high malignancy, poor prognosis and resistance to chemotherapy is one of the leading cancer-associated death in the United States. The proposed agent showed significant Gem accumulation in heated tumor relative to free Gem. Gd labeled TSLnp (Gd-TSLnp) show contrast in ex vivo tumor tissue. The Gd-TSLnp show increased T1 contrast in vivo with an implanted tumor compared to Gd and targets the tumor tissue.
Purpose
Detection of tumors 10 mm or less has been difficult, though MRI has proven to be promising in the diagnosis of early stage tumors (1). Gd chelates have found immense applications in tumor visualization and vascular imaging (2); nonetheless, this approach suffers from rapid extravasation into the extracellular compartment. This translates into a very narrow window for image acquisition. Gemcitabine (Gem) is a drug often used to treat PCa (3). Gem however suffers from lack of tumor specificity, poor membrane permeability and biochemical instability. External trigger-release liposomes such as thermosensitive liposomes have on the other hand the potential to overcome obstacles traditionally seen with liposomes for therapeutic use by providing triggered drug release under conditions of mild hyperthermia. Here, we report on the development of a novel thermosensitive liposomal nanoparticle (TSLnp) capable of carrying Gem and Gd for drug delivery and MRI tracking (4).Methods
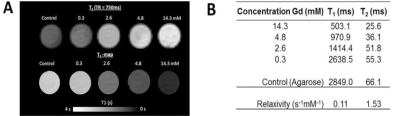
TSLnp and Gd-TSLnps were prepared as previous reported (4-6). For MRI phantoms, Gd-TSLnps were diluted in ratios of 1:1, 1:5, 1:10 and 1:100. Each dilution was mixed with equal parts of 2% agarose and injected into micro-capillary tubes. MRI was performed on a 21.1T (7) magnet equipped with a Bruker Avance III spectrometer (Bruker, Billerca, MA). A 10-mm birdcage coil was used for phantom and ex vivo tissue imaging. All nanoparticle phantoms were imaged in unison. Measurements were set up to quantify R1 (1/T1) and R2 (1/T2) relaxation rates using SE-RARE sequence with 100x100μm in-plane resolution, and 1-mm slice thickness. Nine incrementing repetition times (TR=26–15000ms) and 16 incrementing echo times (TE=10–160ms) were used for T1 and T2 measurements. Regions of interest (ROIs) for each respective dilution were used to calculate R1 and R2. The relaxivity (mM-1s-1) was calculated as a function of Gd concentration. For ex vivo tumor imaging a T1-weighted SE sequence was used with 50x50μm in-plane resolution. TR was incremented between 200–5000ms using TE=15ms. T1 maps were generated with ParaVision 5.1. For in vivo experiments, the animals were sedated and implanted with 5x106 PCa (MiaPaCa-2) cells in right hind limb. Prior to MRI, the animals were sedated with isoflurane and injected intraperitoneal with either Gd or Gd-TSLnp just before insertion into the magnet. To probe contrast and relaxation in vivo, ParaVision 6.0.1 was used together with a 32-mm birdcage coil. T1 maps were acquired with a SE-RARE sequence using TE=6ms and six incrementing TRs (170-4000ms) at 250x210μm in-plane resolution. Dynamic signal-to-noise ratio (SNR) changes were measured with a Turbo SE-RARE sequence using TE/TR=6/1500ms and 90x90μm in-plane resolution and 1-mm slice thickness. Both acquisitions took 7 min.Results
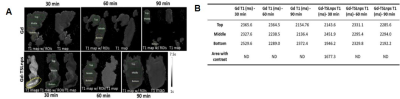
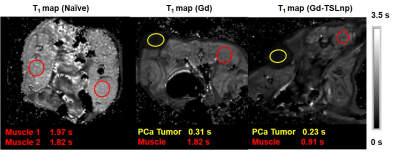
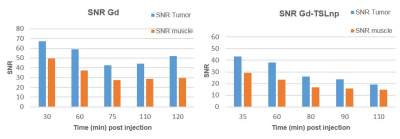
In this study numerous characterizations of the Gem-TSLnp and Gd-TSLnp was made (4). The tissue distribution of both Gem-TSLnps and Gd-TSLnps were significantly greater compared with free Gem and Gd. Gem-TSLnps showed significant Gem accumulation in heated tumor relative to free Gem. Despite Gem-TSLnps dose being half of free Gem dose, antitumor efficacy of Gem-TSLnps was comparable to that of free Gem (4). The Gd-TSLnp showed increased contrast with increasing concentration in agarose (Fig. 1A). Longitudinal relaxivity (r1) was 0.11 s-1mM-1 while r2 was determined to 1.53 s-1mM-1(Fig. 1B). In fixed ex vivo tumor tissue no contrast is seen for any time point after injection with Gd while the Gd-TSLnp show clear hyperintense contrast in the tumor excised after 30 min (Fig. 2A). The T1 contrast did not remain in tumors at 60 and 90 min post injection. To test the in vivo contrast, mice with PCa cells in the hind limb were injected with either Gd or the Gd-TSLnp. T1 maps 50 min post injection show that Gd-TSLnp generate 800 ms lower T1 relaxation in the tumor compared to animals injected with Gd suggesting higher uptake with the Gd-TSLnp (Fig 3). Dynamic SNR measurements show the lack of specificity with free Gd by its decrease and then increase of SNR again at 110 min (Fig 4). This likely indicates in the release from the tumor followed by re-uptake.Conclusion
This study shows that TSLnps can be used as a drug delivery system for poor membrane permeable drugs and provide MRI contrast in the tumor. The TSLnps is targeting the PCa to deliver both Gem and Gd. To fully evaluate the contrast enhancement capability of Gd-TSLnps, future studies aim to optimize TSLnps for higher Gd payload. We also aim to institute a direct injection technique in the vertical magnet using an in situ tail vein infusion line to probe the full dynamic changes of MR contrast after Gd-TSLnp injection.Acknowledgements
All animal experiment were approved by FSU and FAMU animal care and use committee. The authors acknowledge the financial assistance provided by the NIH/RCMI #5G12MD007582-32 and NIH/NCI #5P20CA192990-03. A portion of this work was performed at the US National High Magnetic Field Laboratory, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490.References
1. Hanada K, Okazaki A, Hirano N, Izumi Y, Teraoka Y, Ikemoto J, Kanemitsu K, Hino F, Fukuda T, Yonehara S. Diagnostic strategies for early pancreatic cancer. J Gastroenterol 2015; 50(2):147-54.
2. Ketan B Ghaghada, Murali Ravoori, Divya Sabapathy, James Bankson, Vikas Kundra, Ananth Annapragada. New dual mode gadolinium nanoparticle contrast agent for magnetic resonance imaging. PLoS One 2009; 4(10):e7628.
3. Liu X, Situ A, Kang Y, Villabroza KR, Liao Y, Chang CH, Donahue T, Nel AE, Meng H. Irinotecan delivery by lipid-coated mesoporous silica nanoparticles shows improved efficacy and safety over liposomes for pancreatic cancer. ACS Nano 2016; 10(2):2702-15.
4. Kevin Affram, Ofonime Udofot, Mandip Singh, Sunil Krishnan, Renee Reams, Jens Rosenberg, Edward Agyare. Smart thermosensitive liposomes for effective solid tumor therapy and in vivo imaging. PLoS One 2017; 12(9):e0185116.
5. Udofot O, Affram K, Israel B, Agyare E. Cytotoxicity of 5-fluorouracil-loaded pH-sensitive liposomal nanoparticles in colorectal cancer cell lines. Integr Cancer Sci Ther 2015; 2(5):245-52.
6. Ghaghada K, Hawley C, Kawaji K, Annapragada A, Mukundan S. T1 relaxivity of core-encapsulated gadolinium liposomal contrast Agents—Effect of liposome size and internal gadolinium concentration. Academic Radiology 2008; 15(10):1259-63.
7. Fu R, Brey WW, Shetty K, Gor'kov P, Saha S, Long JR, Grant SC, Chekmenev EY, Hu J, Gan Z, Sharma M, Zhang F, Logan TM, Bruschweller R, Edison A, Blue A, Dixon IR, Markiewicz WD, Cross TA. Ultra-wide bore 900 MHz high-resolution NMR at the national high magnetic field laboratory. J Magn Reson 2005; 177(1):1-8.
Figures