3031
Neurovascular coupling to D2/D3 partial agonist antipsychotic drug occupancy using simultaneous PET/fMRI1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States
Synopsis
Drug-receptor interactions are the basis of signal modulation in the brain, yet, in vivo mechanisms of the action of many drugs are not well understood. In this study, we characterize the in vivo profile of a current third generation antipsychotic drug at the D2/D3 dopamine receptor using simultaneous PET and fMRI. The results are compared to full D2/D3 antagonists and agonists profiles and show that functional differences can be distinguished with occupancy-matched fMRI responses.
Introduction
The binding of a drug or neurotransmitter to a receptor is the basis of neuronal signal transmission and modulation in the brain. Neurovascular coupling to receptor occupancy has been shown to occur for antagonist and agonist drugs specific to the D2/D3 dopamine receptor system using simultaneous receptor-specific PET and fMRI measurements [1,2]. In this study, we extend this concept to current third generation antipsychotic drugs that are classified as partial agonists at the D2/D3 dopamine receptor. Our goal was to characterize the in vivo functional response at D2/D3 receptors of partial agonists in the context of full D2/D3 antagonists and agonists using simultaneous PET/fMRI.Methods
Dynamic [11C]raclopride PET (specific to D2/D3 dopamine receptors) and fMRI were acquired in two anesthetized non-human primates (rhesus macaque) on an integrated PET/MR scanner. Three different doses (0.02, 0.05, 0.1 mg/kg) of the antipsychotic aripiprazole were injected intravenously as a within-scan challenge at ~35 minutes and/or 70 minutes after radiotracer injection. Comparisons were made to two full antagonists (prochlorperazine, raclopride) and two agonists (ropinirole, quinpirole), two of which are clinically relevant (prochlorperazine, ropinirole), with the others being preclinical drugs. Gradient-echo EPI (1.3mm isotropic resolution, TR=3s, TE=23ms) was acquired throughout the dynamic PET acquisition of 100 minutes. Before each scan, iron oxide was injected to improve fMRI contrast and detection power [3]. fMRI data were analyzed with the GLM and cerebral blood volume (CBV) changes were derived. PET data were analyzed with a simplified reference tissue model (SRTM) that included a term for dynamic binding changes and used the cerebellum as the reference tissue in order to determine receptor occupancy [4]. A neurovascular coupling model previously introduced [2] was used to predict functional responses from drugs that exhibit partial agonist and antagonist properties.Results
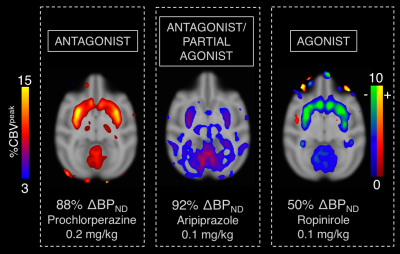
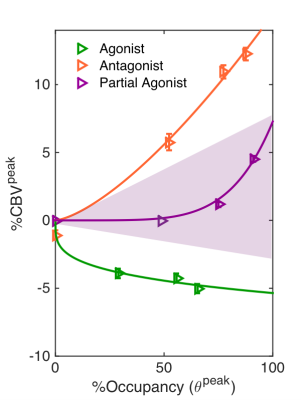
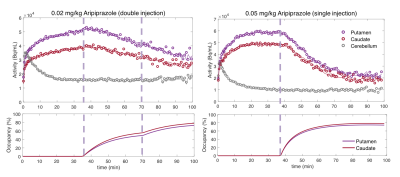
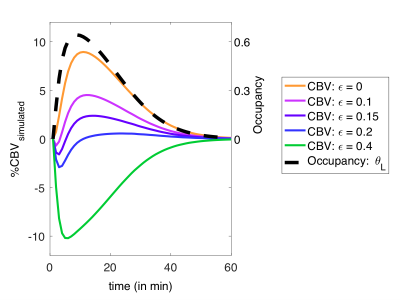
The highest doses of all administered drugs gave the largest D2/D3 receptor occupancy values as measured by [11C]raclopride PET data and corresponded to the largest CBV change for each drug in the striatum. Figure 1 shows the voxelwise CBV maps from the highest dose of aripiprazole in comparison to the two clinically relevant full agonist and antagonist responses, together with the simultaneously acquired occupancy measurement. Interestingly, the atypical antipsychotic aripiprazole showed an overall positive CBV response that was markedly lower in magnitude, despite higher D2/D3 receptor occupancy, compared to the full ant-/agonist. The full agonists consistently showed a negative response and full antagonists a positive CBV response in the striatum. Figure 2 shows a direct comparison of CBV values for each occupancy. D2/D3 occupancy values for the three doses of aripiprazole were 49%, 74% and 92% in the putamen. Simultaneously acquired %CBV values were not significant for the lowest dose of aripiprazole, but showed positive %CBV of 1.2% and 4.5% for the two higher doses in the putamen. Figure 3 shows time-activity curves for the two doses of aripiprazole. A double injection of 0.02 mg/kg aripiprazole separated by 35 minutes led to a similar overall occupancy compared to a single injection of 0.05 mg/kg but could be clearly distinguished in kinetic modeling as separate occupancy curves. Figure 4 shows simulation results from a neurovascular coupling model that demonstrate how efficacy of drugs affects the CBV response in relation to a simulated occupancy timecourse and that partial agonists would be expected to show a diminished magnitude response that can show either long-lasting positive or short-lived negative changes.Discussion
The atypical antipsychotic that is classified as a partial agonist in vitro could be characterized as a partial agonist, in agreement with a neurovascular coupling model. Together with the results from full agonist and antagonists, this brings together a comprehensive dataset that shows that the combination of PET/fMRI can be used to classify D2/D3 drugs according to their in vivo pharmacodynamic profile. Our in vivo results agree with classifications from in vitro studies, but will be especially useful for drugs that are classified as partial agonists or atypical and may not have a defined functional response in vivo. In addition, the timing and route of drug administration can play a major role in its functional effects. Our results also demonstrate advantages and limits simultaneous PET and fMRI datasets that are dependent on timescales and signal specificity. While we investigated D2/D3 receptors, the fMRI response from non-specific drugs such as aripiprazole could also be modulated by other receptors. Further experiments with other partial agonists or clinical antipsychotics may reveal further insight on in vivo drug function that may be invaluable for predicting therapeutic effects of drugs.Acknowledgements
NIH grant support: K99DA043629. This work also involved the use of instrumentation supported by NIH grants P41EB015896, P30DA28800, S10RR026666, S10RR017208, S10RR022976, S10RR019933.References
[1] Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, et al (2013). Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci U S A 110: 11169–74.
[2] Sander CY, Hooker JM, Catana C, Rosen BR, Mandeville JB (2016). Imaging Agonist-Induced D2/D3 Receptor Desensitization and Internalization In Vivo with PET/fMRI. Neuropsychopharmacology 41: 1427–1436.
[3] Mandeville JB, Marota JJA, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, et al (1998). Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med 39: 615–624.
[4] Lammertsma AA, Hume SP (1996). Simplified Reference Tissue Model for PET Receptor Studies. Neuroimage 4: 153–158.
Figures