3030
PET/MR Platform for Neuroscience in Awake Behaving Non-Human Primates1Psychiatry, University of Wisconsin, Madison, WI, United States, 2Neuroscience, University of Wisconsin, Madison, WI, United States, 3Radiology, University of Wisconsin, Madison, WI, United States, 4Medical Physics, University of Wisconsin, Madison, WI, United States, 5Biomedical Engineering, University of Wisconsin, Madison, WI, United States, 6Waisman Center, University of Wisconsin, Madison, WI, United States, 7Psychology, University of Wisconsin, Madison, WI, United States
Synopsis
Higher-order cognitive functions result from dynamic interactions of distributed networks comprised of anatomically, physiologically, and pharmacologically separate components of the nervous system. To further our understanding the basic mechanisms and functions of such networks, as well as how they are affected by the administration of therapeutic drugs, we have developed a PET-fMRI platform to take simultaneous measurements of neural activity (fMRI), and concentration of dopamine (PET) during the same physiological state, and without the confounding effects of anesthetics. With this platform, we have measured for the first time in a primate brain the effects of administering different doses of methylphenidate on extracellular levels of dopamine and functional connectivity.
Purpose
Higher-order cognitive functions result from dynamic interactions of distributed networks comprised of anatomically, physiologically, and pharmacologically separate components of the nervous system. Neuromodulators such as dopamine (DA), which target vast regions of the cerebral cortex and the basal ganglia, exert their influence via complex feedback networks, which are thought to be affected in psychiatric conditions such as attention deficit hyperactivity disorder (ADHD). The therapeutic drug methylphenidate (Ritalin) blocks the DA transporter, which is situated on neuronal membranes with varying concentration throughout the brain. Increased blocking of DA transporters increases the levels of extracellular DA, which has been found extensively useful in the treatment of ADHD. However, Ritalin’s effects on task proficiency are dose, task, and subject dependent. Like other psychiatric drugs, the healthcare system thus spends considerable effort and expense over weeks to months to determine a subject’s proper Ritalin dosage.
To further our understanding the basic mechanisms and functions of such networks, as well as how they are affected by the administration of therapeutic drugs, we have developed a PET-fMRI platform to take simultaneous measurements of neural activity (fMRI), and concentration of DA (PET) during the same physiological state, and without the confounding effects of anesthetics. To achieve this goal, we utilize an awake-behaving Rhesus monkey preparation and eye-tracking for cognitive testing. The Rhesus monkey has a brain with similar structure to humans, can perform complex cognitive tasks, can be repeatedly imaged with PET and undergo comprehensive pharmacological testing.
Methods
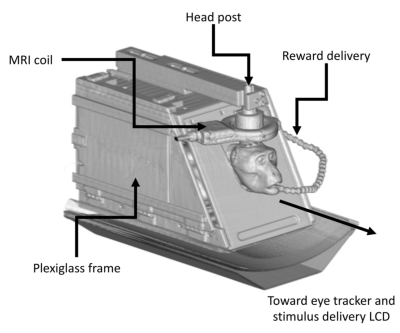
Three awake-behaving monkeys were imaged over multiple sessions in a PET/MR system (Signa PET/MR, GE Healthcare, Waukesha, WI). The subjects received extensive training in a mock scanner, with recorded MR sounds before imaging. Subjects were placed in a custom primate chair in the upright position, then tilted forward into the sphinx position (Fig. 1), and their heads restrained with a head post that held a 3-inch surface loop MRI coil. While in the chair, they receive an oral dose of methylphenidate (0, 1.5, 3, or 6 mg/kg) mixed with fruit juice 45 min before the start of the scanning session. fMRI data acquisition parameters were: TR=1.6s TR, TE=20.5ms, 2.67x2.67x3mm voxel size, and flip=70degrees. Structural MRI was also performed (T1w BRAVO, MPRAGE and T2 CUBE). Additional maps of B0 field were acquired for distortion correction of functional MR data. Prior to MR, ~3 mCi [18F]-fallypride (FAL) was injected as a single bolus. Dynamic PET imaging of D2 dopamine (DA) was conducted over 180 minutes, concurrent with MRI acquisition, and reconstructed with the following parameters: time-of-flight TOF-OSEM, 256x256 matrix, 28 subsets, 2 iterations, 4mm post filter, 60 second frame duration. During data acquisition the monkeys were presented with a dot at the center of a computer screen placed 90cm in front of the bore of the scanner. Eye position was recorded with an eye-tracker (S.R. Research, Ottawa, CA). fMRI data were preprocessed with fieldmap and motion correction, highpass filtering, and smoothing. A a conventional seed-based correlation approach was used to compute resting state connectivity and identify networks. PET data were processed in a manner similar to fMRI, including motion correction, signal normalization to cerebellum, and smoothing. Regions of interest were fitted to a model to assess Fallypride binding potential (BP).Results / Discussion
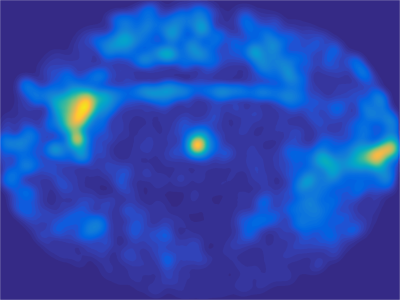
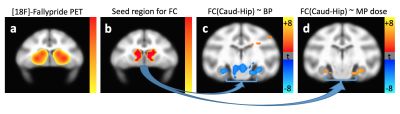
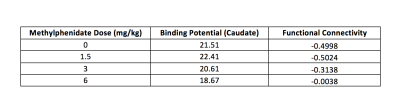
Data from one representative subject are presented. Figure 2 illustrates the oculomotor behavior of the animal during a typical resting state fMRI session. Although no reward was provided, the animal spent most of the time looking at the central dot. Figure 3 illustrates the effects of methylphenidate on [18F]-Fallypride PET binding potential and fMRI connectivity strength. [18F]-Fallypride PET showed greatest uptake in the striatum (Figure 3a), indicating the high density of D2 receptors. Further analyses showed that the binding potential in the caudate, a subregion of the striatum, was significantly associated with methylphenidate (MPH) dose (p<0.05). The functional connectivity between the caudate (Figure 3b) and bilateral hippocampus was significantly associated with both [18F]-Fallypride binding potential (Figure 3c) and MPH dose (Figure 3d, Table 1).Conclusion
We have developed a platform for simultaneous measurements of brain connectivity with fMRI, neurotransmitter levels with PET, and eye-tracking for monitoring behavior in an awake monkey preparation. With this platform, we have measured for the first time in a primate brain the effects of administering different doses of methylphenidate on extracellular levels of dopamine and functional connectivity. This platform opens up many novel avenues for the study of brain function. We will extend our studies from resting state fMRI to task-based fMRI, include measurement of cognitive function, incorporate additional PET tracers, and add sophisticated pharmacological and electroceutical interventions.Acknowledgements
No acknowledgement found.References
No reference found.Figures