3026
Hybrid Liver Multiparametric MRI and F18-FDG PET/MR in Diagnosing and Staging of Intrahepatic Cholangiocarcinoma: An initial Experience1Radiology, Mayo Clinic in Arizona, Scottsdale, AZ, United States, 2Hematology/Oncology, Mayo Clinic in Arizona, Scottsdale, AZ, United States
Synopsis
Intrahepatic cholangiocarcinoma (ICC) is an uncommon biliary tract malignancy with an unfavorable prognosis given its complicated clinical and imaging manifestations. In this preliminary study, we investigated the role of hybrid liver mpMRI and F18-FDG PET/MR in diagnosing and staging of ICC. Our preliminary data show promising value of this “one-stop” imaging modality in providing complementary morphological and functional information in detecting viable tumor burden, defining nodal and distant metastasis utilizing both MRI and PET molecular imaging biomarkers.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is an uncommon biliary tract cancer, but the second most common primary hepatic malignancy after hepatocellular carcinoma. ICC has various morphological presentations and histological types, and is therefore a diagnostic and therapeutic challenge. Accurate diagnosis and staging of ICC are of great clinical importance as surgical resection is considered the only potential cure. Contrast enhanced liver multiparametric MRI (mpMRI) with MRCP has been the imaging modality of choice in delineating tumor extent and associated biliary duct obstruction for this primary malignancy. In addition, F18-FDG PETCT has been shown to be a valuable tool in staging of ICC by detecting nodal and distant metastasis, as well as evaluating the metabolic status of the primary lesion. As such, combining excellent soft tissue contrast with functional molecular imaging information, the novel PET/MR system has great potential for imaging of hepatobiliary malignancies. In a current small cohort study, we summarized our initial experience for a hybrid liver mpMRI and F18-FDG PET/MR in the evaluation of ICC.Subjects and Methods
We reviewed the PET/MR images of first four patients enrolled in our clinical trial (Figure 1), with expectation of 10 total patients by May 2018. All patients underwent F18-FDG PET/MR scan on a 3T PETMR scanner 60 minutes after intravenous administration of 10 ± 1 mCi FDG. The hybrid F18-FDG PET/MR protocol consisted of two parts: 1) torso PET/MR survey and, 2) the focused liver mpMRI. During the torso PET/MR survey, 4 to 5 bed positions covering from vertex of skull to thigh are performed in 20-25 minutes. MR based attenuation correction (AC) and four segmented T1 weighted DIXON-LAVA sequences (water, in-/oppose-phase and fat) were acquired simultaneously and reconstructed using time-of-flight (TOF) method. The focused liver mpMRI was performed at one bed position; and the protocol consisted of multi-planar T1 and T2 weighted sequences, diffusion weighted imaging (DWI) with b value at 0, 50, 500 and 1000 respectively, as well as multiphase post contrast T1-weighted images (arterial, portal venous, equilibrium +/- hepatobiliary phases). During the focused liver mpMRI acquisition, a simultaneous PET task was also performed, including an MRAC series and 8-minute PET data acquisition, to ensure accurate PET/MR fusion and increase lesion conspicuity.
Imaging analysis: The Torso PET/MR survey series were reviewed by a dual board certified nuclear radiologist on MIM Vista workstation. The maximum standard uptake value (SUVmax) of hepatic lesion, lymph node and distant metastases were measured. The focused liver mpMRI images were reviewed on PACS by an experienced abdominal MR radiologist. Consensus diagnosis of each lesion was reached by both radiologists.
Results
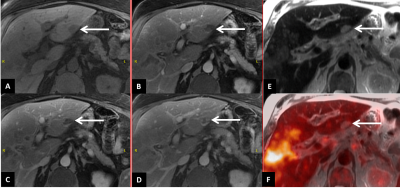
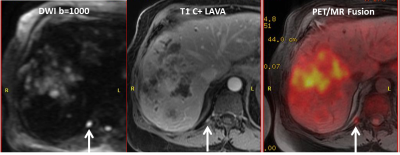
Intrahepatic lesions: There were 10 consensus identified malignant foci (3 primary, 1 recurrent and 6 satellite lesions) demonstrating both abnormal MR imaging features and increased FDG uptake (SUVmax 4.1-14.6). The two primary lesions exhibited higher FDG avidity (SUVmax >10) compared to recurrent and satellite lesions (SUVmax 4.1-7.9). Another primary lesion after radiation therapy showed smaller size on PET compared to MRI (Figure 2). One lesion initially indeterminate on MRI demonstrated no FDG uptake and was diagnosed as hemangioma after consensus analysis (Figure 3). In addition, Gadoxetate disodium enhanced MRI detected two tiny satellite lesions (less than 0.5 cm in dimension) which were too small to characterize on PET.
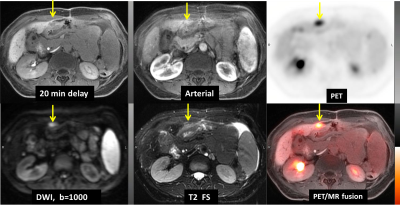
Nodal metastasis: There were greater than 10 FDG-avid lymph nodes (SUVmax 4.1-11.1) identified on PET images regardless of their dimension. While on MRI, without aid of the DWI, only 4 of those lymph nodes greater than 1 cm in short axis were identified (less than 40%); and with aid of DWI, this number increases to 9 (Figure 4).
Distant metastasis: All distant metastases, including peritoneal carcinomatosis (Figure 5) and multiple hypermetabolic lung nodules were visualized on F18-FDG PET/MR images only.
Discussion
Recent retrospective study indicated that the SUVmax of hepatic lesion is significantly related to TNM staging and prognosis of primary ICC. 1 In addition, administration of gadoxetate disodium has shown added value in detection and characterization of tumor burden to F18-FDG PET/MR scan.2 F18-FDG PET/CT is outstanding in detection of nodal and distant metatstasis.3 Our initial experience concurs with the above studies that liver mpMRI and FDG PET provided complementary information in diagnosing and staging of ICC. Interdisciplinary communication plays a crucial role in comprehensive evaluation of both morphological and functional imaging findings with increased reader’s confidence.Conclusion
F18-FDG PET/MR is an excellent “one-stop” imaging modality. The complementary information derived from mpMRI and PET molecular imaging biomarkers is able to aid in the initial staging and subsequent treatment response assessment for the clinical course of ICC.Acknowledgements
NoneReferences
1. Wang ZF, Lan XL, Xiao YR, et al. [Correlation between TNM staging of primary cholangiocarcinoma and the maximum standard uptake value of (18)F-2-deoxy-D-glucose positron emission tomography with computerized tomography]. Zhonghua Yi Xue Za Zhi. 2017 Oct 24;97(39):3104-3107. doi: 10.3760/cma.j.issn.0376-2491.2017.39.013.
2. Kirchner J, Sawicki LM, Deuschl C, et al. 18 F-FDG PET/MR imaging in patients with suspected liver lesions: Value of liver-specific contrast agent Gadobenate dimeglumine. PLoS One. 2017 Jul 6;12(7):e0180349. doi: 10.1371/journal.pone.0180349
3. Lee Y, Yoo IR, Boo SH, et al. The Role of F-18 FDG PET/CT in Intrahepatic Cholangiocarcinoma. Nucl Med Mol Imaging. 2017 Mar;51(1):69-78. doi: 10.1007/s13139-016-0440-y. Epub 2016 Aug 6
Figures