3018
18F-FDG PET/MRI Allows Early Detection of Foam Cell Formation and Fat Deposition in Hemorrhagic Myocardial Infarctions1Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Lawson Health Research Institute, London, ON, Canada
Synopsis
Inability of macrophages (MΦ) to switch from pro-inflammatory (M1, glycolytic) to anti-inflammatory (M2, oxidative) phenotype can lead to increased glucose transporter 1 (GLUT1)-mediated glucose metabolism, decreased fatty acid (FA) beta oxidation, increased intracellular lipid accumulation, and MΦ-to-foam cell transformation. Recent studies in the field of chronic venous leg ulcers have shown that iron-overloaded MΦ fail to switch from M1 to M2 phenotype. In this study we hypothesized that inability of iron-overloaded MΦ to switch from M1 to M2 phenotype underlies fatty degeneration of hemorrhagic myocardial infarction via MΦ lipid accumulation and their transformation into foam cells.
INTRODUCTION
While pro-inflammatory (M1) macrophages are highly dependent on glucose as an energy substrate, anti-inflammatory (M2) macrophages are preferentially fueled by fatty acid (FA) β-oxidation. Failure to switch from M1 to M2 macrophage phenotype can lead to prolonged inflammatory response accompanied by increased glucose transporter 1 (GLUT1)-mediated glucose metabolism, decreased FA beta oxidation, increased intracellular lipid accumulation, and macrophage-to-foam cell transformation. Recent studies in the field of venous leg ulcers have shown that chronic iron-overloading of macrophages can prevent the physiologic switch from glycolytic M1 to oxidative M2 phenotype.
In atherosclerosis imaging, 18F-fluorodeoxyglucose (18FDG)-PET has emerged as a promising tool for visualizing the early stage of foam cell formation in vulnerable plaques, depending on the extent of macrophage infiltration into the atherosclerotic lesion. A recent in-vitro study has demonstrated that 18FDG accumulation was increased during foam cell formation, but the uptake was decreased to the control level after the cells had differentiated to foam cells. These findings suggested that 18FDG PET is capable of detecting the early stage of foam cell formation in atherosclerosis.
A growing body of evidence now shows iron-laden scar tissue of hemorrhagic myocardial infarction (MI) is preferentially populated by M1-polarized macrophages. In this study we investigated the metabolic profile of hemorrhagic MI in both acute and chronic settings using 18FDG PET/MRI. We hypothesized that inability of iron-loaded macrophages to switch from glycolytic to oxidative phenotype underlies fatty degeneration of hemorrhagic MI via macrophage lipid accumulation and their transformation into foam cells.
METHODS
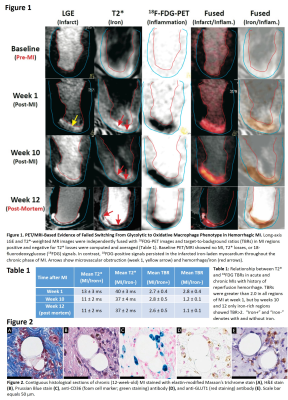
Canines (n=3) were subjected to 3-hour occlusion of the LAD artery, followed by reperfusion. All dogs underwent in-vivo T2*, LGE and 18FDG-PET/MR imaging at baseline, week 1, and week 10 post-MI in a hybrid 3T PET/MRI scanner. To suppress myocardial uptake of FDG, animals were fasted, treated with 2000 units of heparin, and infused with 20% IV fat emulsion. Twenty minutes after lipid infusion, 18F-FDG PET images were acquired simultaneously with T2* and LGE images (in that order). T2* weighted (multiple gradient-echo, TR=12ms, 6TEs=2.0ms–9.5ms with ΔTE=1.5ms, flip angle=10°, BW=931Hz/pixel) and LGE images were acquired along the short-axis direction covering the entire LV. The imaging resolution for all MR scans was 1.4 x 1.4 x 6 mm3. The same imaging protocol was repeated for post-mortem in-situ imaging at week 12 post-MI after which explanted hearts underwent histopathological evaluation. Long-axis LGE and T2*-weighted MR images were independently fused with 18FDG-PET images and target-to-background ratios (TBRs) in MI regions positive and negative for T2* losses were computed and averaged. All qualitative image analyses were performed using the cvi42 (Circle Cardiovascular Imaging Inc) or Image J (NIH).RESULTS
Hemorrhagic MIs exhibited a glycolytic phenotype throughout the chronic phase of MI as evidenced by increased 18FDG metabolism on weeks 1, 10, and 12 post-MI (Figure 1). TBRs were greater than 2.0 in all regions of MI at week 1, but by weeks 10 and 12 only iron-rich regions showed TBR>2 (Table 1). Retention of the glycolytic phenotype in macrophages undergoing foam cell transformation was confirmed by intense immunoreactivity for GLUT1 (Figure 2).DISCUSSION
Our data suggest that prolonged inability of iron-laden macrophages within MI to switch from M1 to M2 phenotype promotes macrophage-to-foam cell transformation in hemorrhagic MIs. These findings underscore a key role for iron in fat deposition in hemorrhagic MIs.