3014
Cardiac MRI with the Siemens Terra 7T System: Initial Experience and Optimization of Default Protocols1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital, Wuerzburg, Germany
Synopsis
The demand for the application of Ultra-High Field (B0≥7T) MR-scanners in cardiovascular MRI grows permanently despite of technical challenges increasing significantly with the static magnetic field strength. We report initial experience with the new 7T system Siemens Magnetom™ Terra for acquiring MR-images of the human heart. A standard workflow for cardiac assessment has been developed and tested in N=18 healthy volunteers in single transmit mode. Currently CINE scans with 14-17 slices covering up to 35 heart phases are well suited for clinical volumetric heart function characterization. Diagnostic image quality can be provided for subsequent volunteers.
Introduction
Ultra-High Field (UHF) imaging (B0≥7T) allows significantly increased spatial resolution in human brain MRI scans (~100µm). Despite technical challenges due to B0 and B1 inhomogeneity, which increase with field strength, there is a strong interest and demand for the application of UHF scanners in cardiovascular MRI. Delivery of the new 7T system Siemens Magnetom™ Terra (Siemens Healthineers, Erlangen, Germany) to first customers has recently begun. In this manuscript, we report our initial experiences with default sequences and protocols available on the scanner for acquiring MR-images of the human heart in-vivo both, for clinical and research purposes.Methods
With approval of the local ethics committee, N=18 healthy volunteers (14-female) were scanned using 1TX/16RX thorax coil (MRI Tools, Berlin, Germany). Patient age range was 20-41 years, and body weight 53-102 kg. For triggering both the integrated ECG and an external acoustic triggering system (MRI Tools, Berlin, Germany) were used for synchronization of image acquisition to the heart. The system providing a more stable trigger signal was chosen for gating for each volunteer. Clinical images were obtained using cardiovascular (CV) GRE and bSSFP (TrueFISP) pulse sequences for CINE acquisition. However, at 7T only GRE-readout can be utilized in practice with typical settings for resolution and number of slices due to SAR-limitations. Sequence parameters were TE=3.57ms, FOV=320x295mm2, matrix size=256x222, slice thickness=6 mm. GRAPPA acceleration with R=2 and 3 was applied. In CINE mode, 6-11 segments and 20-35 cardiac phases were measured with retrospective triggering. Short axis CINE stacks for volumetric evaluation varied in slice number (14-17) and were applied using multiple-breath-hold concatenations (maximal breath-hold time 13s). Since no dedicated cardiac shim setting was available, B0 shimming was performed using “Brain” default shim settings providing both minimal initial TE=1.0ms and ∆TE=2.0ms. The shim volume was chosen to cover myocardial tissue only. B0 mapping was done using triggered multiGRE-sequence with phase reconstruction; parameters were FOV=300×300mm, matrix size=128×128, TR = 100ms, FA=25°, TE=1.7/2.8/4.0ms. B1-mapping was performed using standard scanner-side mapping sequence (“b1map”) based on turbo-FLASH readout with 90° preparation pulse, FOV=450x235mm2, matrix size=256x128.
Results
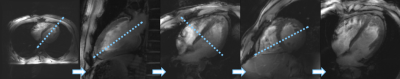
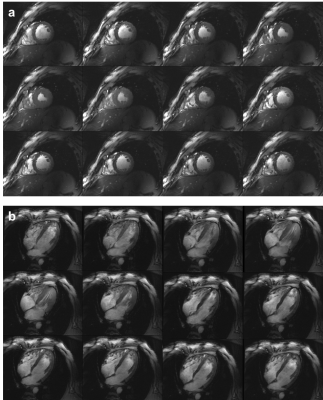
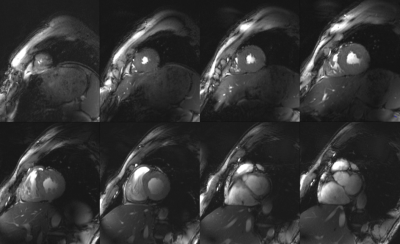
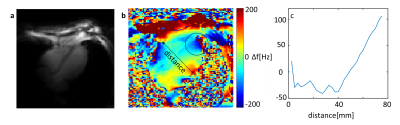
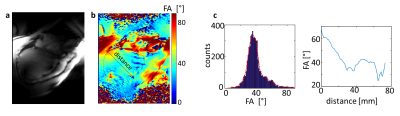
Figure 1 demonstrates the standard workflow for routine cardiac planning views using gradient echo sequences in order to acquire a standard short and long axis view. Based on these planes, standardized short and long axis gradient echo CINE sequences can be acquired (Figure 2). For volumetric evaluations, a multislice CINE stack of the short axis view with full heart coverage is performed (Figure 3). Blood/myocardium contrast was found to be 5±1.5. From 18 volunteers, in 6 the ECG-triggering was used, and in 12 the acoustic triggering. Thus appropriate gating was achieved in all volunteers. Figure 4 shows the B0-maps of short and long axis views. Characteristic B0-inhomogeneity on the interface between myocardial tissue and lungs can be seen. Figure 5 displays typical B1-maps of the heart. The flip angle variation is relatively high as was expected at 7T. The inter-quartile range of FA distribution reaches 15°. The anterior-posterior flip-angle-gradient is up to 10°/cm. No major side effects related to either static magnetic field or RF irradiation were registered.Discussion
The CINE images are well resolved, both spatially and temporarily over the full cardiac cycle. Motion artifacts still visible in heart phases outside of systolic and diastolic “sweet spots” do not create major problems for visual analysis of the images as well as for quantitative analysis using segmentation of myocardium and extraction of typical heart function parameters (e.g., stroke volume). Taking into account the T1-relaxation factor blood/myocardium contrast matches reported literature values1. B0-maps show that, as expected, the maximal susceptibility induced inhomogeneity in the myocardial tissue is caused by interface to the lung parenchyma, which has essentially lower average density (0.1 – 0.2 g/cm3). The homogeneity of FA should be improved by employing a parallel transmit array (pTX) instead of 1TX array. The major difficulties of this study were related to the customization of the localizing pulse sequences. The typical fast transversal “scout” protocol providing whole thorax coverage and good contrast for the heart tissue at 3T (HASTE) is not applicable at 7T due to SAR limitations. As an alternative, the CV protocol was used with multiple breath-hold concatenations. Subsequent sequences for further cardiac planning had to be based on gradient echo readout instead of TrueFISP.Conclusion
A clinical cardiac MRI workflow has been established at the new 7T Siemens Terra system. Considering the field strength, image quality was surprisingly good without major methodological improvement. Further improvement will be required to use 7T MRI to its full possibilities.Acknowledgements
Financial support: German Ministry of Education and Research (BMBF, grants: 01EO1004, 01E1O1504).References
1. Thalhammer, C., et al., Two-Dimensional sixteen channel transmit/receive coil array for cardiac MRI at 7.0 T: Design, evaluation, and application. Journal of Magnetic Resonance Imaging, 2012. 36(4): p. 847-857.Figures