3013
In Vivo Hyperpolarized MRI Reveals Metabolic Changes Following Treatment with Mildronate in the Control and Diabetic Heart.1University of Oxford, Oxford, United Kingdom
Synopsis
L-carnitine acts as a buffer of acetyl-CoA units in the mitochondria, as well as facilitating transport of fatty acids. Mildronate can block the biosynthesis of L-carnitine and its uptake by inhibiting CPT-1. The purpose of this study was to investigate the effect of Mildronate treatment on cardiac function and metabolism in the healthy and the diabetic rat heart. We show that daily injections of Mildronate can alter cardiac metabolism in the in-vivo diabetic and healthy rat heart, without any functional changes, and surprisingly Mildronate can increase flux through pyruvate dehydrogenase. Such studies will allow a better understanding of the interactions between metabolism and function in the diabetic heart and may provide new insight into novel therapeutics.
Introduction
One of the main causes of mortality in type 1 diabetic patients is cardiovascular disease [1]. Improving our understanding of the metabolic changes that occur in the diabetic heart may be key to understanding the pathophysiology of the cardiac effects of diabetes. One key metabolic effect observed in the diabetic heart is an increase in fatty acid oxidation. The anti-ischemic drug, Mildronate has been shown to reduce fatty acid oxidation through inhibition of the mitochondrial fatty acid transporter, CPT1. Mildronate inhibits CPT1 by reducing both the biosynthesis of L-carnitine and its reabsorption in the kidneys [2,3]. In this study we wanted to investigate the metabolic and functional effects of Mildronate treatment on the healthy and diabetic rodent heart to see if it would result in a metabolic shift away from fatty acid oxidation towards glucose oxidation, with potential functional benefits.Method
12 male Wistar rats (~250g) were split into two groups. In one group, rats were injected with streptozotocin (STZ, 55 mg/Kg) to induce a model of type 1 diabetes and in the other group, rats were injected with citrate buffer (CTR) to act as controls. Two weeks after injection, daily intra-peritoneal injections of either saline or Mildronate (100mg/kg/day) were initiated and continued for three weeks. Following treatment, the animals were anesthetized with isoflurane and ECG gated 13C MR pulse-acquire cardiac spectra were acquired over 60s following injections of hyperpolarised [1-13C]pyruvate and [2-13C]pyruvate (Injections separated by ~ one hour; repetition time 1s; excitation flip angle 15°; sweep width 13,021Hz; acquired points 2,048; frequency centred on the C1 pyruvate resonance). Acquired spectra were summed over 30s from the first appearance of pyruvate and analysed with JMRUI [4] for metabolic assessment of the heart. In addition, eight-ten short-axis slices (slice thickness:1.6mm, matrix size:128×128, TE/TR:1.67/4.6ms, flip angle:15°, number of averages:4) were acquired with a CINE-FLASH sequence and analysed with ImageJ for assessment of cardiac function. Following imaging the hearts were excised and Langendorff perfused with an initial normal-flow of 20min, followed by 30min of low-flow (0.4ml/min) ischemia and re-perfused again with normal-flow for 20 min as described by Heather et al. [5].Results
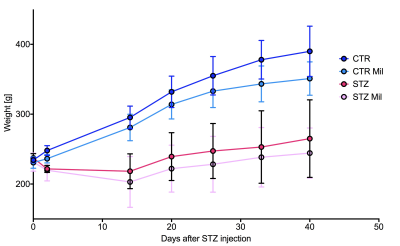
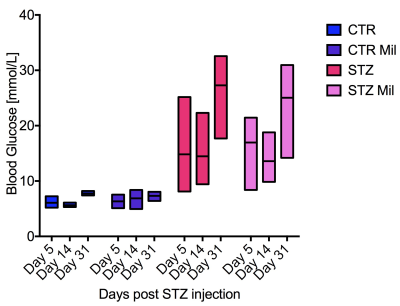
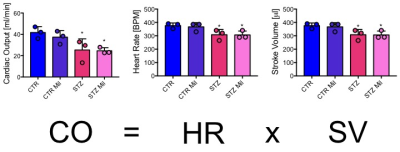
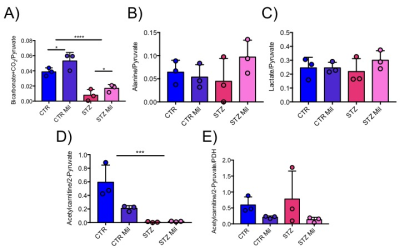
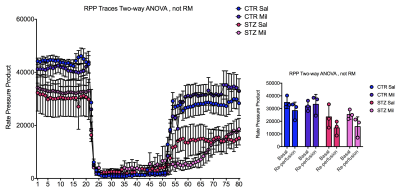
All animals gained weight over the treatment period. The diabetic rats gained less weight than the controls and the Mildronate treated rats gained less weight than the saline treated animals (Fig. 1). The saline and Mildronate treated STZ groups showed similar blood glucose progressions (Fig. 2). Cardiac output, heart rate and stroke volume were decreased in both STZ groups compared to the two control groups (Fig. 3A) with no significant changes seen with Mildronate treatment. Flux through pyruvate dehydrogenase (PDH), as indicated by the bicarbonate + CO2 to pyruvate ratio, was reduced in the saline and Mildronate treated STZ animals compared to the control animals (Fig. 4A). However, despite this reduction, PDH flux was significantly higher with Mildronate treatment in both groups. Incorporation of the 13C label from pyruvate into both lactate and alanine was not significantly changed in any group (Fig. 4BC). The incorporation of [2-13C]pyruvate into acetylcarnitine was reduced in both diabetic groups with no significant effect of Mildronate treatment (Fig. 4D). However, after normalisation to PDH flux, the Mildronate treated animals showed lower 13C label incorporation into acetylcarnitine when compared to saline treated controls (Fig. 4E). The rate pressure product (RPP) was reduced in the two diabetic groups, however there was no significant effect of Mildronate treatment (Fig. 5).Discussionand Conclusion
No differences in weight gain or blood glucose levels were observed between the Mildronate and saline treated groups. Decreased cardiac function in the form of reduced cardiac output, stroke volume and heart rate were observed in all diabetic animals irrespective of treatment with Mildronate. However, despite not seeing any functional differences, Mildronate treatment led to metabolic changes that were observed with hyperpolarized MRS. Mildronate increased pyruvate dehydrogenase flux in the heart in both the control and diabetic groups. Incorporation of the 13C label into acetylcarnitine was reduced in the Mildronate treated groups, confirming the L-carnitine lowering effect of Mildronate as observed previously [2,3]. Further studies are needed to understand the mechanism through which Mildronate increases PDH flux. Such studies will allow a better understanding of the interactions between metabolism and function in the diabetic heart and may provide new insights into novel therapeutics.Acknowledgements
No acknowledgement found.References
[1] Aaron M Secrest et al. "Mortality in Type 1 Diabetes" Diabetes Care, 33,2573-2579, 2010.
[2] Maija Dambrova et al.
"Mildronate: Cardioprotective Action Through Carnitine-Lowering
Effect" TCM Vol 12, No. 6, 2002.
[3] Edgars Liepinsh et al
" Mildronate treatment alters ϒ-butyrobetaine and L-carnitine concentrations in healthy
voluneteers" JPP, 63, 1195-1201, 2011.
[4] Vanhamme L, van
den Boogaart A, Van Huffel S. Improved method for accurate and efficient
quantification of MRS data with use of prior knowledge. J Magn Reson
1997;129:35-43
[5] Heather L, Pates K, Atherton H, Cole M. Ball D et al. " Differential translocation of the fatty acid trasnport, FAT/CD36, and the glucose trasnporter, GLUTr, coordinates changes in cardiac substrate metbaolism during ischemia and reperfusion" Circ. 6, 2013,1058-66.
Figures