3007
Towards Black Blood MRI of the Heart and Large Vessels at 7.0 T: Assessment of Inversion Pulse Quality in Phantom Experiments and In-Vivo Applications1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine (MDC) in the Helmholtz Association, Berlin, Germany, 2Technische Universität, Berlin, Germany, 3Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Inversion recovery prepared cardiac black blood RARE techniques (IR-RARE) are routinely applied at clinical field strengths while still facing numerous challenges at 7.0 T. Realizing the clinical importance of IR-RARE and the benefits of UHF, this study aims at the design of a double inversion recovery prepared imaging technique at 7.0 T. The inversion efficiency and signal suppression efficiency of hyperbolic secant (HS4 and HS8) inversion pulses were analyzed in phantom experiments. First preliminary in-vivo applications using the implemented HSn pulses showed promising results.
Introduction
Inversion recovery (IR) prepared black blood RARE/fast spin echo techniques [1] are applied at clinical field strengths for morphological imaging of the heart and large vessels [1], for the assessment of myocardial edema [2, 3], and for noninvasive myocardial tissue characterization [4]. The SNR gain inherent to imaging at 7.0 T promises to enhance spatial and temporal resolution. At 7.0 T, the use of IR prepared RARE imaging is challenged by transmission field (B1+) inhomogeneities, prolonged T1 relaxation, and increased radiofrequency power deposition. Realizing the clinical importance of black blood preparation for cardiac imaging and recognizing the benefits of UHF-MR, this study aims at the design of a double inversion recovery (DIR) prepared RARE imaging technique for cardiac dark blood imaging at 7.0 T.Methods
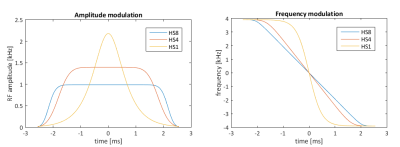
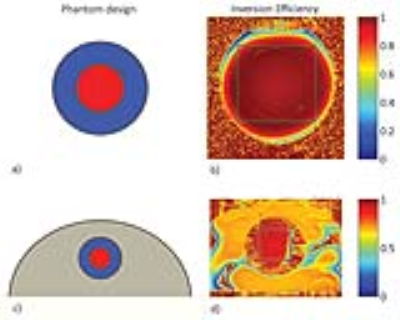
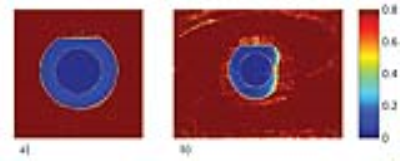
In order to enable DIR, two hyperbolic secant (HS4 and HS8) pulses were designed using MATLAB [5] and implemented into a RARE pulse sequence (Fig.1). A water-CuSO4 phantom was built in-house for the assessment of the inversion pulse quality at 7.0 T (Siemens, Erlangen, Germany). The assessment included inversion efficiency quantification ($$$IE=M_z(TI=0)/M_0$$$) [6] using one inversion pulse and the calculation of signal suppression efficiency. Two different transceiver coil arrays were used: a 1TX/24RX head coil (Nova Medical, Inc., MA, USA) (setup A) and a 16TX/RX cardiac RF coil array (MRI.TOOLS GmbH, Berlin, Germany) (setup B). For setup B, the water-CuSO4 phantom was placed into the center of a torso agarose phantom (Fig.2d) to mimic the location of the heart within the body. The slice profile of the inversion pulses was tested for setup B using a DIR-prepared sequence, with the slice selective pulse set to be orthogonal to the imaging slice. Inversion pulse parameters (pulse length tp, peak amplitude A0, and bandwidth) were varied as part of the quality assessment and chosen to be tp=25 ms, bwdth=11 kHz, and A0=450 V. Following the phantom study, in-vivo experiments in healthy volunteers were performed.Results
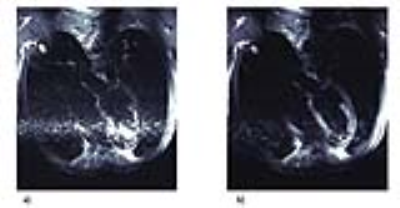
Phantom experiments revealed similar inversion efficiencies when using the HS4 and HS8 pulses (Fig.2). The IE for setup A using one inversion pulse was IE=-0.97 with a standard deviation σ=0.03, while it was IE=-0.91 and σ=0.11 for setup B. Signal suppression of the blood compartment was successful with Mz(TInull)/M0=0.01 and σ=0.01 using the head RF coil and Mz(TInull)/M0=0.02 with σ=0.02 using the cardiac RF coil (Fig.3). The slice profile of the inversion pulses was measured for setup B using DIR and revealed Mz(blood)/Mz(slice)=0.04 (Fig.4). Figure 5 shows dark blood four-chamber views acquired in an exemplary healthy volunteer. The contrast between myocardium and blood was calculated to be $$$ν=(I_{myo}-I_{blood})/(I_{myo}+I_{blood})=0.31$$$ without dark blood preparation compared to $$$ν=0.64$$$ when using DIR (Fig.5).
Discussion
While the inversion efficiency of the HS4/HS8-pulses was sufficient with IE=-0.97 and σ=0.03 when using the head RF coil, the quality of the inversion pulses using the cardiac RF coil array was 6% lower with IE=-0.91 and σ=0.11. It was observed that magnetization inversion over a larger field of view requires a significantly higher B1+ so parameters like the inversion pulse length and peak amplitude needed to be adjusted accordingly. However, longer pulse lengths induce T2 decay during the pulse duration. Also, the peak amplitude is constrained due to SAR and limited peak RF power. A successful in-vivo implementation therefore requires a careful balance of IE, SAR, and T2 relaxation effects. Preliminary in-vivo experiments showed an improvement of myocardium-blood-contrast by 106%. Since TI depends on TR which is determined by the length of the cardiac cycle, heartbeat irregularities lead to insufficient blood suppression, enhanced flow artifacts, and irregular acquisition times. Current work therefore focuses on an optimization of sequence parameters to facilitate an application for irregular heartbeats. Furthermore, a hybrid RARE-EPI [7] pulse sequence will be used in future in-vivo applications. This Combined Acquisition Technique (CAT) benefits from shorter acquisition times since the use of EPI echoes shortens the echo train length, while still achieving high image quality [7]. With this approach cardiac and respiratory motion artifacts can be minimized.Conclusion
In this study, the efficacy of HS4/HS8 pulses was examined for IR-prepared dark blood RARE MRI at 7.0 T using a 1TX/24RX channel head coil and a 16TX/RX cardiac RF coil array. The magnetization inversion is more successful when using the small size head RF coil, but first in-vivo implementations using the designed inversion pulses were promising. Current work focuses on the adjustment of sequence parameters in order to balance SAR and transmit voltage and to support data acquisition for irregular heartbeats.Acknowledgements
No acknowledgement found.References
[1] Simonetti, O.P., et al., "Black blood" T2-weighted inversion-recovery MR imaging of the heart. Radiology, 1996. 199(1): p. 49-57.
[2] Abdel-Aty, H., O. Simonetti, and M.G. Friedrich, T2-weighted cardiovascular magnetic resonance imaging. Journal of Magnetic Resonance Imaging, 2007. 26(3): p. 452-459.
[3] h-Ici, D.O., et al., Cardiovascular magnetic resonance of myocardial edema using a short inversion time inversion recovery (STIR) black-blood technique: diagnostic accuracy of visual and semi-quantitative assessment. Journal of Cardiovascular Magnetic Resonance, 2012. 14: p. 22.
[4] Heinrichs, U., et al., Myocardial T2* mapping free of distortion using susceptibility-weighted fast spin-echo imaging: a feasibility study at 1.5 T and 3.0 T. Magn Reson Med, 2009. 62(3): p. 822-8.
[5] MATLAB, The MathWorks, Inc., Natick, Massachusetts, United States.
[6] Kellman, P., D.A. Herzka, and M.S. Hansen, Adiabatic inversion pulses for myocardial T1 mapping. Magnetic resonance in medicine, 2014. 71(4): p. 1428-1434.
[7] Hillenbrand, C., et al., MR CAT scan: a modular approach for hybrid imaging. Magnetic Resonance Materials in Physics, Biology and Medicine, 2000. 10(3): p. 183-199.
[8] Rodgers, C.T., et al., Inversion recovery at 7 T in the human myocardium: measurement of T1, inversion efficiency and B1+. Magnetic resonance in medicine, 2013. 70(4): p. 1038-1046.
[9] Bernstein, M.A., K.F. King, and X.J. Zhou, Handbook of MRI pulse sequences2004: Elsevier.
Figures