3005
Preliminary Investigation of Extravascular Fluid Transport along Arterial Adventitia of Human Lower Extremity1Cardiology Division, Beijing Hospital, Beijing, China, 2CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Chinese Academy of Sciences, Beijing, China, 3MR Research China, GE Healthcare, Beijing, China, 4Radiological department, Beijing Hospital, Beijing, China, 5School of Future Technology, University of Chinese Academy of Sciences, Beijing, China
Synopsis
Extravascular fluid transport have been reported both in human and animal studies during recent decades. Our previous work demonstrated a long-distance extravascular fluid transport which is consisted of oriented fibrous connective tissues in venous adventitia, arterial adventitia and dermis of amputated lower extremities. To further explore the pattern of fluid transport along lower extremity arteries, we implemented contrast enhanced MRI in volunteers and tracked the longitudinal contrast agent transportation. The periarterial regions near tibia showed high signal intensity after contrast agent administration suggest an unexplored extravascular fluid transport. This study may provide a novel diagnosis method of PAD.
Introduction
A phenomenon of extravascular fluid transport has been reported for decades, which is hinted in researches on ischemic stroke, Alzheimer’s disease (AD), perivenous connective tissues in rabbits, perivenous pathways in mouse brain and tumors 1-6. In human anatomic studies, a long-distance extravascular fluid transport in ankle dermis was displayed in amputated lower leg extremities 7. Histological analysis testified the transport pathways consisted of oriented fibrous connective tissues and comprised three anatomic sites: venous adventitia, arterial adventitia and dermis. In the imaging studies of volunteers, the paramagnetic tracer can be used to visualize similar longitudinal nonvascular transport pathways in the extremities when it was injected into the distal ends of toes or fingers. However, the pattern of periarterial fluid transport along lower extremity arteries has never been explored by MR technique in in vivo study. Therefore, we utilize contrast enhanced MR imaging and MR vessel wall imaging for the investigation of periarterial fluid transport in lower extremity of healthy volunteers.Methods
Seven male healthy volunteers (average age 28±3) with no inducement and history of peripheral artery disease (PAD) were recruited and received contrast enhanced MRI investigation of the right feet and legs. Each MR examination was carried out on a 3T DISCOVERY MR750 scanner (GE) and with a standard lower extremity coil at Beijing Hospital in China. The MRI protocol was approved by Beijing Hospital and all the volunteers had signed informed consent before MRI scanning and injection of Gd-DTPA (Magnevist, Bayer Healthcare) at volumes of 0.5 mL (the diluted concentration was 46 mg/mL) into the hypodermics tissues of the distal end of the second toe. The MR protocol consisted of two QIR8 based axial arterial wall imaging and one T1-weighted imaging. Two QIR sequences with the same settings (TI1/TI2/TR/TE: 430/150/11/900 ms, In-plane resolution: 0.59 X 0.59 mm, Thickness: 1.0 mm, Total 15 slices, Field of view: 150 X 150 mm) were implemented before and 8 min after the contrast agent administration, respectively. To track the pathway of this transport behavior, T1-weighted imaging using 2D GRE sequence (TR/TE: 2.8/14ms, In-plane resolution: 1.75 X 1.75 mm, Thickness: 1.0 mm, Total 248 slices, Field of view: 280 X 280 mm) was applied.Results
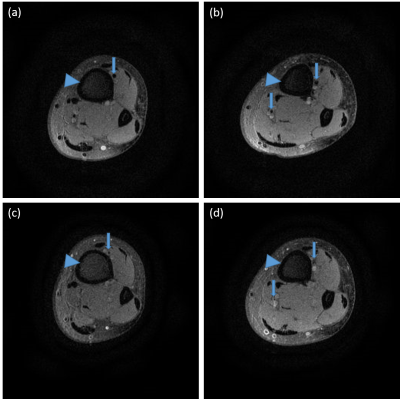
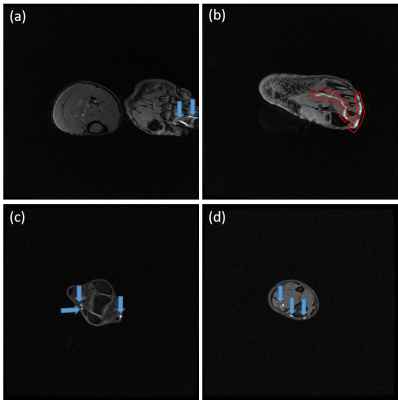
Before Gd-DTPA administration, the low extremities of volunteers were scanned by QIR sequence. Periarterial regions near tibia show low signal intensity shown in Fig. 1 (a) and (b). Eight minutes after contrast agent administration, the regions show high signal intensity shown in Fig. 1 (c) and (d). To track the longitudinal contrast agent transportation, T1-weighted imaging using 2D GRE sequence was implemented at volunteers who were treated the same as the aforementioned experiment. The contrast agents were observed not only in the region of artery but also in the subcutaneous region as shown in Fig. 2.Discussion
In common physiological knowledge, the arterial vessel transports blood centrifugally through its endothelial lumen. However, we found that the periarterial tissues around the tibia artery were enhanced by the paramagnetic tracer administrated from the hypodermic tissues of the distal end of lower leg. The enhancement was not the results of blood transport through the vascular vessels because the same positions around fibular artery was not enhanced concurrently. As to the vasa vasorum, no anatomic data record that these nourishing vessels are longitudinally distributed and covering arterial adventitia to form venous capillary plexus along arterial vessel tree. Moreover, an injection site on foot dorsum was used in control group and no enhancement has been found in periarterial tissues. Thus, together with the previous results, these findings strongly suggest that there is a novel extravascular fluid transport along the arterial adventitia of human lower legs.Conclusion
The current data further verify the periarterial fluid transport along the lower extremity artery and strongly suggest an unexplored systemic extravascular fluid transport in human body. Their physiological and pathophysiological functions need further extensive studies. Meanwhile, the imaging technique of arterial adventitia of extremities may provide a novel method for diagnosis of PAD with arteriosclerosis obliterans.Acknowledgements
This study was supported by the 973 Program (No. 2015CB554507) and the 121 Program of Beijing Hospital (No. 121-2016002).References
1. Arbel-Ornath M, Hudry E, Eikermann-Haerter K, et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models[J]. Actaneuropathologica, 2013, 126(3): 353-364.
2. Iliff J J, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β[J]. Science translational medicine, 2012, 4(147): 147ra111-147ra111.
3. Iliff J J, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI[J]. The Journal of clinical investigation, 2013, 123(3): 1299.
4. Louveau A, Smirnov I, Keyes T J, et al. Structural and functional features of central nervous system lymphatic vessels[J]. Nature, 2015, 523(7560): 337-341.
5. Li H, Chen M, Yang J, et al. Fluid flow along venous adventitia in rabbits: is it a potential drainage system complementary to vascular circulations?[J]. PloS one, 2012, 7(7): e41395.
6. Munson J M, Shieh A C. Interstitial fluid flow in cancer: implications for disease progression and treatment[J]. Cancer management and research, 2014, 6: 317. 7.
7. Li H, Yang C, Lu K, et al. A long-distance fluid transport pathway within fibrous connective tissues in patients with ankle edema[J]. Clinical hemorheology and microcirculation, 2016, 63(4): 411-421.
8. Yarnykh V L, Yuan C. T1-insensitive flow suppression using quadruple inversion-recovery[J]. Magnetic resonance in medicine, 2002, 48(5): 899-905.
Figures